PIERRE ROBIN EUROPE
Welcome to Pierre Robin Europe, a not-for-profit international organization of patients and clinicians that provides support and counseling for patients suffering from the rare disease, Pierre Robin Sequence / Pierre Robin Syndrome. We collaborate with local Pierre Robin Sequence patient groups and clinicians around the world and pursue various goals, including the following:
- To promote communication and increase awareness about the rare disease, Pierre Robin Sequence.
- To share scientifically supported and accessible information about Pierre Robin Sequence with Pierre Robin Sequence patients, their families, and healthcare providers who are treating babies with this rare disease.
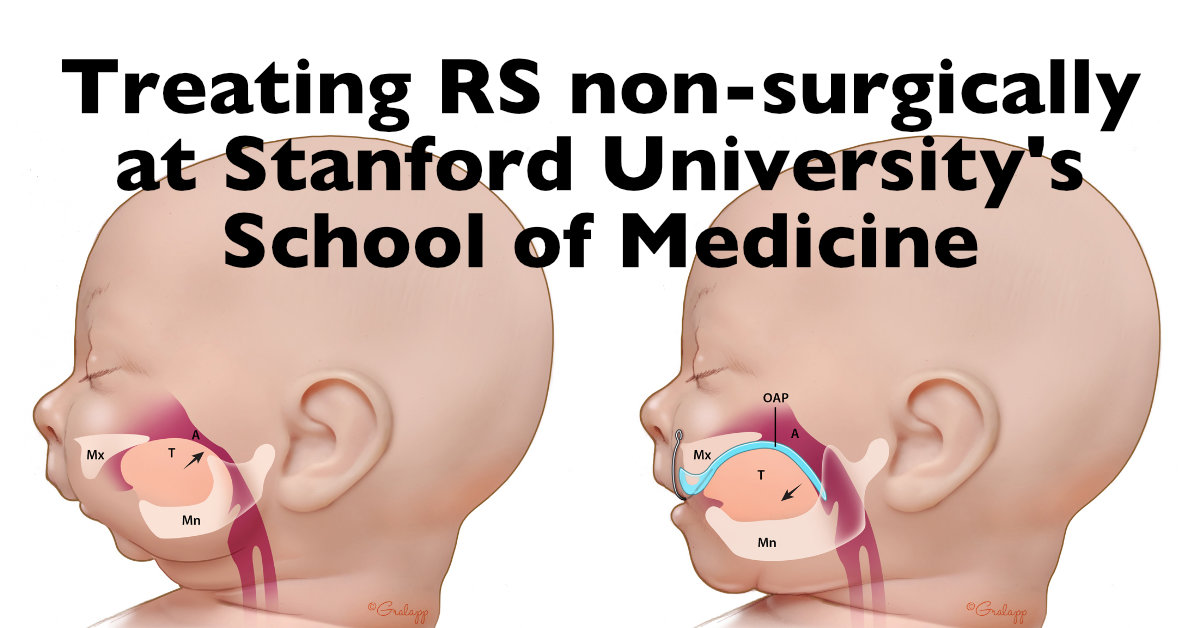
- To provide information on the techniques which currently exist to diagnose Pierre Robin Sequence during the prenatal period; to advocate for the development of a scientifically validated fetal ultrasound screening protocol which can be used by ultrasound professionals around the world to prenatally diagnose Pierre Robin Sequence.
- To increase access to care by explaining to Pierre Robin Sequence patients in the European Union how they can access highly specialized rare disease treatments in other EU Member States using the EU’s 2011 Directive on Patients’ Rights in Cross-border Healthcare, Regulation 883 on the Coordination of Social Security Systems, and the case-law of the Court of Justice of the European Union; to engage with lawyers, academics and patient advocacy groups on the use of these laws; to engage in public outreach and legal action when such laws are not respected.
- To advocate for patient centered care, and the right to access care, not only for the benefit of babies suffering from Pierre Robin Sequence, but for the benefit of all rare disease patients, wherever they may be.