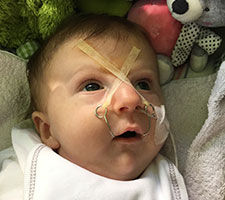
Letter to President Macron
Part 2: Medical Analysis
Medical Analysis
The medical basis of L’Assurance Maladie’s rejection
The statement explaining the medical basis of L’Assurance Maladie’s rejection declares that “the same treatment or an equally effective treatment can be obtained in France without undue delay”.
Is “the same treatment” available in France?
Concerning the first scenario, “the same treatment”: in the email from Dr. Abadie, dated 28 April 2017, Dr. Abadie, the top Pierre Robin Sequence expert in France, admits that:
“Currently, this technique can only be carried out by this German team because it is based on the custom manufacture of an orthosis which has to be set up, monitored and changed by the experienced team.”
French original:
“Cette technique n’est actuellement réalisable que par cette équipe allemande car elle repose sur la fabrication sur mesure d’une orthèse qui doit être mise en place, surveillée et changée par l’équipe qui en a l’expérience.”
As confirmed by Dr. Abadie in writing, the TPP treatment is not available in France.
Furthermore, on Monday 10 April 2017, we discussed our child Lysiane’s health with Dr. Abadie by telephone. Dr. Abadie explained that she knows Dr. Poets in Tübingen, and she knows his work. Concerning the TPP treatment, Dr. Abadie told us, “I know this technique, and I know Professor Poets. It’s a technique which works. It works, it’s undisputable.” Dr. Abadie also confirmed that this medical device, the TPP, is not available in France, in England, or in the United States of America. The TPP device is a medical device custom made for each particular patient by a team of German medical professionals using highly specialised health technology, which requires a particular concentration of expertise, based in Germany. Dr. Abadie explained to us: “The TPP treatment is not a generalized treatment. It’s very local, it’s done only in Germany. You have to go to Germany for this treatment.”
Once again, the TPP treatment is not available in France, and no treatment identical to the TPP treatment is available in France.
Is there “an equally effective treatment” in France?
The “same treatment” is not available in France; this is a straightforward fact. Does France offer an “equally effective treatment”? Various invasive surgical techniques are available in France for treating babies with Pierre Robin Sequence. One example of these invasive surgical techniques is mandibular distraction osteogenesis. Mandibular distraction osteogenesis essentially involves fracturing the baby’s lower jaw, and then surgically enlarging the lower jaw using a series of metal rods and screws – all in an effort to reduce the baby’s upper airway obstruction.
Another invasive surgical technique which France offers to treat babies suffering from Pierre Robin Sequence is labioglossopexy. This surgical procedure involves sewing the end of the baby’s tongue to the baby’s lower lip; the idea is that by sewing the baby’s tongue down, it will prevent the baby’s tongue from moving up and blocking the baby’s upper airway, or from moving at all. Labioglossopexy – sewing a baby’s tongue to the baby’s lip – is a hideous procedure; it resembles something from the Middle Ages, not medicine from the 21st century. Furthermore it often fails to do its job; either the flesh of the baby’s tongue rips, or the flesh of the baby’s lip rips, creating flesh wounds and possible infection – and allowing the baby’s tongue to return to its dangerous vertical position (glossoptosis), where it obstructs the upper airway.
We believe that if France’s doctors informed French mothers and French fathers that there was a safe and effective alternative to these painful and invasive and potentially risky surgical procedures – if they were told that an oral device, the TPP device, has been medically proven to consistently and successfully eliminate the baby’s upper airway obstruction, without any surgical intervention whatsoever – then at least some of these French families would forego the surgery, and opt instead for the TPP treatment.
In her email dated 28 April 2017 for L’Assurance Maladie, concerning the treatments France offers for babies suffering from Pierre Robin Sequence, Dr. Abadie avoids any mention of these or other surgical techniques which are offered in France. Instead she indicates that France’s strategy for treating babies with Pierre Robin Sequence is to attach these babies to ventilator machines:
“In France we have an effective alternative strategy for the treatment of upper airway obstruction, as is shown in our publications: Continuous Positive Airway Pressure for Upper Airway Obstruction in Infants with Pierre Robin Sequence.”
Is it true that when compared to the TPP treatment, France’s ventilation assistance – attaching the baby to a ventilator machine – is “equally effective”?
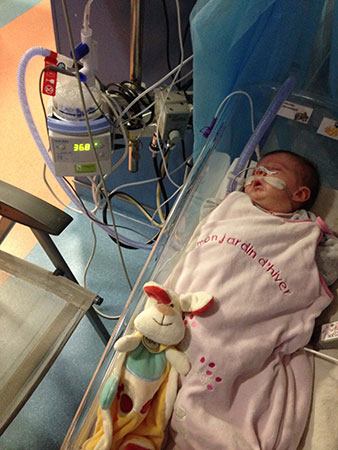
- The TPP treatment, by unblocking the baby’s throat, corrects the underlying anatomical problem, Pierre Robin Sequence’s upper airway obstruction; this allows the baby to breathe naturally and independently, on her own. Ventilation assistance, on the other hand, simply forces air down the baby’s blocked airway; the baby’s airway, however, remains obstructed, and when the baby is disconnected from the ventilator machine, the breathing difficulties return. Here is Lysiane, struggling to breathe, when she was momentarily disconnected from the ventilator machine, after a month of nonstop 24/7 hospitalization in France, where she was receiving ventilation assistance:
http://avantetapres.com/ - The TPP treatment requires no external equipment; instead the TPP is a small oral device which is worn inside of the baby’s mouth. Ventilation assistance, on the other hand, requires cumbersome hospital equipment, including a ventilator machine, tubes, a facial mask, and electrical cables; this ventilation equipment radically limits the mobility of both the baby and the parents, since the baby has to be attached to the ventilator machine whenever the baby sleeps, both night and day.
- The TPP treatment generally requires 2 to 3 weeks in the hospital; the baby is then discharged from the hospital, and goes home to join the parents. Ventilation assistance generally requires long term hospitalization, which can last for several months, half a year, or longer. The need for long term hospitalization, which ventilation assistance generally requires, has several major disadvantages:
- long term hospitalization comes at a tremendous financial cost, in any healthcare system, regardless of how that healthcare system is managed;
- long term hospitalization exposes the baby to bacterial pathogens in the hospital, which can worsen the baby’s existing health problems; and
- long term hospitalization creates a painful, unnatural, and unhealthy separation between the baby and the parents, and interferes with the bonding process which is crucial for the baby’s healthy development.
- The TPP treatment, by correctly repositioning the baby’s tongue forward, and flat in the mouth, successfully places it in the correct and natural position for nursing; this helps to trigger the baby’s natural and inborn sucking and swallowing instincts. Bottle feeding is thus dramatically improved, because the TPP ensures that the baby’s tongue is, by default, in the correct nursing position. This has been proven in peer reviewed medical studies; the TPP treatment substantially improves and facilitates the feeding process in infants suffering from Pierre Robin Sequence. Ventilation assistance, on the other hand, does nothing to improve or facilitate feeding.
- The TPP treatment, by repositioning the tongue toward the front of the mouth, causes the baby’s tongue to press against the baby’s lower alveolar ridge. There is medical evidence suggesting that this gentle pressure of the baby’s tongue, pressing against her lower alveolar ridge, naturally induces mandibular catch-up growth, thereby reducing the jaw deformities associated with Pierre Robin Sequence, safely and non-surgically. CPAP, on the other hand, has been shown to create a clinically significant risk of actually causing facial deformities in young children, due to the constant pressure the ventilation mask exerts on the growing facial structures of these children. Dr. Brigitte Fauroux, a member of France’s Pierre Robin Reference Centre at Necker Hospital in Paris, published a study here in France showing that 68% of the children observed, who received ventilation assistance, suffered from facial deformities, including global facial flattening and maxillary retrusion.
No objective and impartial medical comparison can reasonably conclude that CPAP, which requires the baby to be connected to a ventilator machine, and the TPP treatment, which achieves crucial medical objectives that CPAP cannot and does not achieve, are “the same or equally effective”.
Below we will provide further details on the points raised above.
Equipment, and patient mobility
In the S2 application we submitted to L’Assurance Maladie, we included a letter from Dr. Christian Poets. In this letter, Dr. Poets explained the following:
“…ventilation comes with several challenges, including the fact that this intervention must be applied almost continuously in (young) infants, as these sleep for most of the day, which substantially reduces the patient’s mobility. In contrast, the TPP, which is placed inside of the baby’s mouth, eliminates the need for any cumbersome external equipment and thus allows parents to move their baby around freely and, most importantly, also at home.”
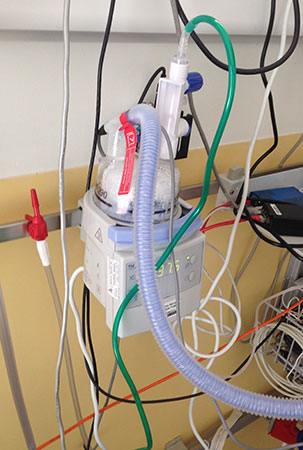
The issue which Dr. Poets is addressing above relates to patient mobility. Ventilation support has to be applied when the baby sleeps – but a baby sleeps most of the day, and most of the night. Thus, a baby receiving ventilation support needs to be connected to the external ventilation equipment almost 24 hours a day. This makes it very difficult for parents to move the baby from one room to another, to take the baby to medical appointments, and so on. The following two images illustrate this difference in the equipment required for ventilation support, as opposed to the TPP treatment, and the effect this required equipment has on the mobility of children and parents:
| Equipment, and its effect on patient mobility | |
|
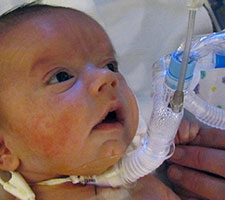
Ventilation assistance
Ventilation equipment; the various air tubes and electrical wires are part of the ventilation equipment. The baby has to be connected to this equipment each time the baby sleeps, which is the majority of the time. |
TPP treatment
The small objects above are examples of the TPP device, which is worn inside of the baby’s mouth. This small oral device is all the equipment our child needs to unblock her upper airway obstruction and allow her to breathe naturally and freely, on her own. There is no need for any external equipment whatsoever, no need for any electrical wires, and no need to remain stationary, anchored to a ventilator unit, and connected to tubes in a hospital’s intensive care unit. |
Long term hospitalization
In the same letter from Dr. Poets, he added the following:
“…ventilation typically requires a long-term hospital stay, which greatly increases the cost of treatment and carries additional risks, particularly contact with pathogenic bacteria (hospital-acquired, i.e. nosocomial, infections). In the present case, the patient, Lysiane Pakter, has already been under constant hospitalization for over 4 weeks. Unfortunately, she continues to require both, ventilation and critical nutritional support. The TPP treatment, on the other hand, requires a hospitalization period which averages two to three weeks; the patient is then discharged home to rejoin his/her parents, who have been trained in handling and maintaining the TPP device on their own.”
Long term hospitalisation is a critical issue. Not only does long term hospitalisation come at a tremendous financial cost, and not only does it expose the baby to bacterial pathogens, but long term hospitalisation also creates a painful, unnatural, and unhealthy separation between the baby, and the baby’s parents. As Lysiane’s parents, we were traumatized by the terrifying circumstances of Lysiane’s dangerous birth, and the discovery that she had a rare disease, and the fact that she had to spend 6 straight days in the Neonatal Resuscitation unit, receiving the highest level of urgent medical care. However, we were further devastated that after Lysiane left the Neonatal Resuscitation unit, the long term hospitalization continued, in the Intensive Care unit – week after week after week after week.
We could not move into the hospital and live with Lysiane in the Intensive Care unit; all we could do was visit her every day. However, every night we had to leave the hospital, and go home without her. This was not good for Lysiane, and it was not good for us, the parents.
Furthermore there was no endpoint in sight; there was no scheduled release date, no estimate from any of the doctors, as to when we would be able to finally bring Lysiane, our newborn baby, home.
On the other hand, in Tübingen, in Germany, after only one week of the TPP treatment, we were able to put Lysiane in a stroller, leave the hospital, and take our baby out into the open air for a walk in the park. With the French treatment, putting Lysiane into a stroller and taking her to the park would have been an impossible dream.
On 21 May 2017, after less than three weeks of the TPP treatment, we were completely discharged from the German hospital. We took Lysiane, our baby, home. We did not need to bring a ventilator machine, air tubes or face masks. From the German hospital’s discharge report:
“After the TPP’s modifications were complete we carried out a repeat sleep study which revealed an MOAI < 1. Clinical signs of upper airway obstruction were eliminated... With regard to her respiratory difficulties, treatment has allowed Lysiane to achieve total independence from ventilation assistance."
The following two images illustrate this important difference: the need for long-term hospitalization, in the case of ventilation assistance – and freedom to leave the hospital, in the case of the TPP treatment.
| Long term hospitalisation | |
|
Ventilation assistance
Above, in France, with ventilation support; five consecutive weeks at the hospital, without ever leaving once, and no release date in sight. |
TPP treatment
Our daughter Lysiane was fitted with a TPP device on 4 May 2017. Six days later, on 10 May 2017, we were able to take Lysiane outside in a stroller for a walk in the park. Lysiane did not need any ventilation equipment, only the TPP device in her mouth, which unblocks her airways, and allows her to breathe naturally and independently, on her own. On 21 May 2017, after less than three weeks of the TPP treatment, we were discharged from the hospital completely. We brought Lysiane, our baby, home. We did not need to bring any ventilator machine, air tubes, or face masks with us. |
The palatal plates of Professor Isabelle James in Lyon
The TPP device we sought in Germany is an example of a type of medical device called a palatal plate. This type of treatment – a palatal plate – is available in France, and is covered by L’Assurance Maladie. This is confirmed by Dr. Abadie in her email dated 28 April 2017, in which she wrote:
“In Lyon, little palatal plates can be proposed by the surgical team of Professor Isabelle James. These palatal plates have no effect on ventilation, but they are able to help some infants to position their tongue during feeding. This technique is not backed by scientific proof, but it exists.”
Original text, in French:
“A Lyon, des petites orthèses palatines peuvent être proposées par l’équipe de chirurgie du Pr Isabelle James. Elles n’ont pas d’effet sur la ventilation mais peuvent aider certains enfants à positionner leur langue pendant la tétée. Cette technique n’a pas fait la preuve scientifique de son efficacité, mais existe.”
In describing the palatal plates of Professor Isabelle James, Dr. Abadie’s goal was to convince L’Assurance Maladie that there is no need for Lysiane to travel to Germany to obtain the TPP device, because this type of treatment, the palatal plate, is readily available in France as well.
We as parents made contact, in France, with the mother of a French baby suffering from Pierre Robin Sequence. Her baby recently received one of the “little palatal plates… of Professor Isabelle James”, which Dr. Abadie is referring to above. The mother told us about the French palatal plate her baby had received, and she sent us photos. This confirmed for us that although the French palatal plates and the German palatal plates both belong to the exact same type of medical treatment, the German palatal plates are, objectively speaking, medically superior to the French palatal plates:
- The French palatal plates do nothing to help Pierre Robin Sequence babies with their potentially life-threatening breathing difficulties; as Dr. Abadie admitted in writing, the French palatal plates have “no effect on ventilation”;
- The German palatal plates do successfully resolve the baby’s breathing difficulties; by successfully resolving the baby’s breathing difficulties, the German palatal plates effectively eliminate the baby’s risk of oxygen deprivation, brain damage, and death; in terms of quality of life, the German palatal plates liberate these babies from mechanical breathing machines, and from long-term hospitalisation, and from painful, risky and invasive surgery;
- The French palatal plates lack peer-reviewed medical studies to demonstrate what benefits they offer, and what risks they create; this too is a fact which Dr. Abadie admitted in writing;
- The German palatal plates are backed by, and benefit from, over ten solid years’ worth of peer-reviewed medical studies; these peer-reviewed medical studies have not only proven the German plates to be highly effective – they have also proven that the German plates are indeed safe for these babies to use.
Thus, while this type of treatment, the use of palatal plates, is offered in France, and is covered by L’Assurance Maladie, the French palatal plates, and the German palatal plates, are not “equally effective”. The German palatal plates are, objectively speaking, medically superior to the French palatal plates.
In conclusion, L’Assurance Maladie is indeed willing to authorise this type of medical treatment, the use of palatal plates, to treat French babies suffering from this specific rare disease, Pierre Robin Sequence. However, L’Assurance Maladie will only authorise the use of palatal plates if two conditions are met. The first condition is that the palatal plates must not resolve the upper airway obstruction and breathing difficulties faced by these babies. If the palatal plates do in fact resolve the upper airway obstruction, and potentially life-threatening breathing difficulties, faced by these babies – as the German plates do – then they will not be authorised by L’Assurance Maladie. The second condition to qualify for authorisation by L’Assurance Maladie is that the palatal plates must not be backed by any peer reviewed medical studies. If the palatal plates are in fact backed by peer reviewed medical studies, confirming their safety, and effectiveness – as the German plates are – then they will not be authorised by L’Assurance Maladie.
It appears that for Dr. Abadie and L’Assurance Maladie, it does not matter how effective the palatal plates are, or whether the palatal plates have been medically tested and scientifically proven. What matters is where the palatal plates come from. If they come from France, they must be good, so L’Assurance Maladie is willing to authorise their use. If they come from Germany, then they must be bad, so L’Assurance Maladie will refuse them. By similar logic, the French government has every right to obstruct French citizens from purchasing BMW and Mercedes cars, because here in France we propose Peugeot and Citroën cars – and Peugeot and Citroën cars should be good enough for any French citizen. Based on EU law, it is difficult to believe that L’Assurance Maladie considers such conduct to be acceptable.
From the perspective of patients’ rights, which includes the right to receive the best possible medical treatment – especially a newborn baby suffering from a rare disease – it would be difficult to conjure up a healthcare policy more irrational or more perverse.
| Palatal plates in France; palatal plates in Germany | |
|
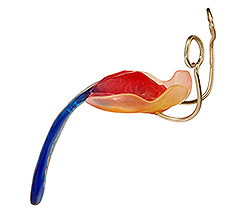
The French palatal plates
In terms of form, these plates do not have any rear extension descending down into the pharynx. In terms of function, these plates do not shift the base of the tongue forward, and do not unblock the upper airway. Dr. Abadie does not mention these important facts in her letter dated 28 April 2017, but she does admit that: “They have no effect on ventilation…” Dr. Abadie explains that these palatal plates: “…are able to help some infants to position their tongue during feeding.” but she admits a lack of scientific proof: “This technique is not backed by scientific proof, but it exists.” |
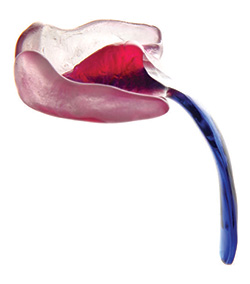
The German palatal plates (the TPP)
The TPP device has a velar extension which descends approximately 2 to 3 centimetres into the pharynx. This velar extension shifts the base of the tongue forward, so that the tongue is in a flat and horizontal position in the baby’s mouth. This unblocks the rear of the baby’s throat, and instantly frees the upper airways. Creating a mould of the baby’s mouth for the purpose of producing a conventional palatal plate is not overly difficult. The velar extension, on the other hand, makes the manufacture of the TPP device complex. The manufacture of a TPP device requires an interdisciplinary team including neonatologists trained in upper airway nasopharyngeal fibre optic endoscopy. These doctors study the interior of the baby’s throat while the baby is wearing the TPP device, to precisely modify and perfect the form, angle and dimensions of the TPP’s velar extension, in order to unblock the baby’s upper airway. The TPP treatment, available only in Germany, has been definitively proven in numerous peer reviewed medical studies over more than 10 years. The effectiveness and safety of the TPP device is medically proven. |
CPAP: the “equally effective” treatment
In her email dated 28 April 2017 for L’Assurance Maladie, Dr. Abadie wrote that:
“In France we have an effective alternative strategy for the treatment of upper airway obstruction, as is shown in our publications: Continuous Positive Airway Pressure for Upper Airway Obstruction in Infants with Pierre Robin Sequence.”
Also, during our 10 April 2017 telephone call, when Dr. Abadie was comparing the TPP treatment to CPAP, she said that ”les résultats sont les mêmes” (“the results are the same”).
We as parents were already familiar with ventilation assistance, because Lysiane was receiving ventilation assistance during her hospitalisation in Lyon. She received it shortly after her birth, and she had to be put back on ventilation assistance less than a week after being admitted to the Intensive Care unit, as a result of continuing respiratory difficulties. Thus when we were speaking with Dr. Abadie by telephone on 10 April, we were genuinely surprised that she would characterize the TPP treatment and CPAP as being medically equivalent. The TPP device is a small device which is worn in the mouth. CPAP, on the other hand requires a considerable amount of serious hospital equipment.
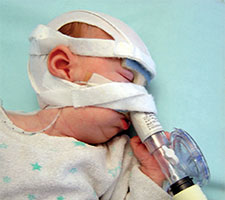
Below, a Pierre Robin Sequence baby receiving ventilation assistance through a custom made mask. The mask must be connected by tubes to external equipment, a ventilator machine, which radically limits the baby’s mobility.
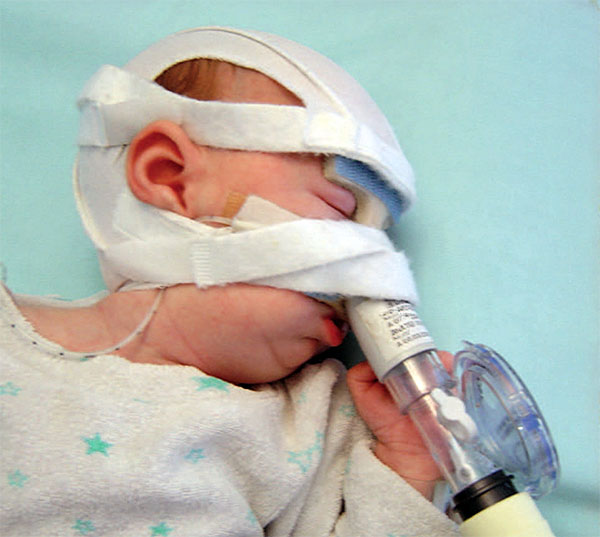
In addition to the fact that CPAP requires serious hospital equipment, including a mask, tubes and a ventilator machine, the CPAP equipment has to be used whenever the baby sleeps. This was something of a surprise for us. During the phone call, seeking clarification, we asked Dr. Abadie, “so the baby has to wear the CPAP mask at night?” She responded, “when the baby sleeps” – in other words, yes, at night, all night, but also during the day – whenever the baby is sleeping. This suddenly made it clear to us that ventilation, whether provided in the hospital, or provided through in-home hospitalisation, was far more intrusive than we had originally thought. Ventilation, even for moderate upper airway obstruction, has to be applied “when the baby sleeps” – but our baby, like most babies, doesn’t just sleep at night – she also sleeps during the day. This means that with CPAP, we would have to keep Lysiane connected to external CPAP equipment, or have external CPAP equipment available just next to her in case she suddenly takes a nap, almost around the clock, 24 hours a day.
This fact is confirmed in the medical study which Dr. Abadie wrote, and which she referred to in her letter to L’Assurance Maladie – “Continuous Positive Airway Pressure for Upper Airway Obstruction in Infants with Pierre Robin Sequence”. This medical study, authored by Dr. Abadie, states the following:
“Continuous positive airway pressure was used continuously in the severe upper airway obstruction group with the aim of decreasing continuous positive airway pressure use to sleep periods only over a 1 – to 2-week period. The moderate upper airway obstruction group was treated with noninvasive continuous positive airway pressure during sleep periods only.”
The need to depend on the external CPAP equipment both at night and during the day is a major disadvantage of CPAP, when comparing CPAP with the TPP treatment. However, this is only one of the several major disadvantages of CPAP, when compared with the TPP treatment.
CPAP and facial deformities
Another major disadvantage of CPAP when compared with the TPP treatment is that CPAP creates a serious danger of “facial side effects” – i.e. physical deformities of the face. This is a risk which Dr. Abadie never mentioned over the telephone during our 10 April conversation, and which she also failed to mention in her email dated 28 April for L’Assurance Maladie. However, the risk of facial side effects when applying CPAP is medically documented. Not only is the risk of facial side effects medically documented, but it is documented in a medical study authored by a doctor who works with Dr. Abadie, Dr. Brigitte Fauroux. What makes this almost incredible is that Dr. Brigitte Fauroux happens to be the major advocate of CPAP for babies with Pierre Robin Sequence at Dr. Abadie’s Pierre Robin Reference Centre at Necker Hospital in Paris.
Dr. Fauroux’s study is called “Facial side effects during noninvasive positive pressure ventilation in children” (Fauroux B, Lavis JF, Nicot F, et al. Facial side effects during noninvasive positive pressure ventilation in children. Intensive Care Med. 2005; 31:965–969). The study states that:
“In children facial deformity can occur due to the pressure applied by the mask on growing facial structures. The aim of the present study was to evaluate the facial side effects of nasal masks use for NPPV in children.”
“Global facial flattening was observed in 68% of the patients and concerned the forehead (43%), malar area (38%), and maxilla (28%). One or two anatomical regions were concerned in 37% and 18% of the patients, respectively. A concave face was observed in 12% of the patients.”
“A maxillary retrusion was observed in 37% of the patients.”
“This observational study underlines the high prevalence of facial side effects of nasal mask use in children.”
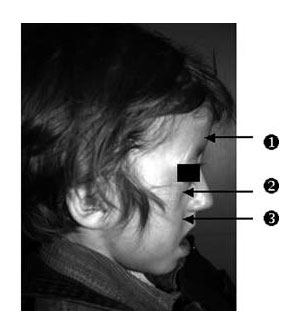
Below is evidence from Dr. Fauroux’s study, “Facial side effects during noninvasive positive pressure ventilation in children”. The photo below shows a child who suffered from severe deformities as a result of receiving NPPV. The child below suffered from flattening of the forehead (1), flattening of the malar area (2), and flattening of the maxilla (3), all due to the pressure exerted by the ventilation mask on the growing bones of the child’s face.
Dr. Fauroux’s study points out that the high risks of facial side effects from NPPV was independent of whether the NPPV mask was a commercial mask, or a custom-made mask which was specially designed for babies:
“Custom-made masks were used in all the patients younger than 6 years of age and in older patients in the case of nontolerance of commercial masks… Global facial flattening was not related to the type of mask, presumably because both commercial and custom-made masks exert pressure on the same anatomical area.”
In spite of the fact that custom-made masks were used for all patients younger than 6 years old, the very young patients still suffered from a high risk of facial side effects from NPPV. Apparently, the physical deformities caused by the masks were a result of the pressure the masks exerted on the face – and this pressure is necessary to create a proper seal, regardless of which type of mask is being used, custom-made or commercial.
CPAP, Maxillary Retrusion, > 10 hours per day
Global facial flattening was not the only side effect of NPPV; maxillary retrusion was another deformity caused by NPPV. In informal language, maxillary retrusion is a caving in of the upper jaw, where the baby’s upper teeth will eventually grow.
Below is more evidence from Dr. Fauroux’s study, “Facial side effects during noninvasive positive pressure ventilation in children”. The photo below shows a child who suffered from maxillary retrusion, again as a result of receiving NPPV.
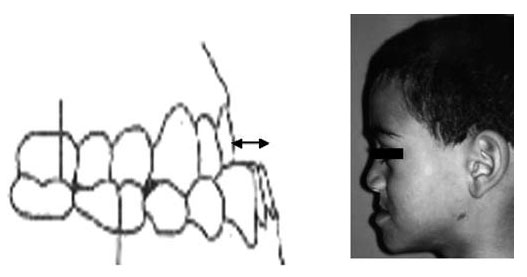
For babies like Lysiane, who suffer from Pierre Robin Sequence, maxillary retrusion is a particularly serious concern, for several reasons.
The first reason maxillary retrusion is a serious matter for our daughter, and for any baby with Pierre Robin Sequence, is that Pierre Robin Sequence is already associated with facial deformities, including micrognathia (a small underdeveloped lower jaw) and retrognathia (an abnormal rear positioning of the lower jaw). The last thing such a baby needs is to be exposed to the serious risk of yet another deformity affecting the jaw, maxillary retrusion.
The second reason maxillary retrusion is so serious is that Dr. Fauroux’s study showed that maxillary retrusion – which was observed in no less than 37% of their patients – was not correlated at all with how many months or how many years the mask was used. Instead, maxillary retrusion was only correlated with the number of hours per day which the mask was worn:
“A maxillary retrusion was observed in 37% of the patients… In univariate and multivariate analyses only daily use (>10 h per day) was associated with maxillary retrusion (OR 6.3, 95% CI 1.3–29.3, p=0.02)… maxillary retrusion was associated with a daily NPPV use exceeding 10 h per day.”
As Dr. Abadie explained, babies suffering from Pierre Robin Sequence would receive NPPV/CPAP “when the baby sleeps”. Babies certainly sleep more than “10 h per day”. This means that our daughter, who already suffers from midface hypoplasia facial deformities, retrognathia and micrognathia, would, with NPPV, also be exposed to a high risk of further deformities, both global facial flattening, and maxillary retrusion. Furthermore, she would be exposed to this high risk of further facial deformities even if she only wore the NPPV mask for a few weeks. This is because, as Dr. Fauroux’s study showed, maxillary retrusion was the result of using the mask for “>10 h per day” – not how many weeks or months or years the NPPV treatment was applied.
This is a very important point which bears repeating: according to Dr. Fauroux’s study, the serious risk of maxillary retrusion is triggered not by months and months of use, but by usage which exceeds 10 hours per day. Pierre Robin Sequence babies suffering from moderate or severe upper airway obstruction would need to wear the mask whenever they sleep, which is definitely more than 10 hours per day.
In the study, Dr. Fauroux presents ideas for preventing the facial deformities caused by NPPV. However, her proposed solutions are themselves quite alarming:
“A decrease in the mask pressure and a reduction in the daily NPPV use could present a preventative measure of these side effects.”
Thus, to reduce the risk of facial deformities caused by NPPV, the patient should simply receive less NPPV per day. How can a baby who is suffering from Pierre Robin Sequence, and who is supposed to wear the mask “when the baby sleeps“, as Dr. Abadie explained, cut back on the daily use of the NPPV mask? Should parents of a baby suffering from Pierre Robin Sequence prevent their baby from going to sleep, in order to reduce the high risk of facial deformities caused by CPAP? No answer is given to this troubling question.
And what happens when the NPPV treatment has indeed actually caused physical deformities – global facial flattening and/or maxillary retrusion – in a given patient? Once again, Dr. Fauroux proposes the same “solution” which she proposed for prevention: simply reduce the daily use of the NPPV treatment:
“Remedial measures for side effects could include a prompt change of the mask in the case of skin injury and the reduction in the daily use of NPPV.”
Dr. Fauroux provides the following conclusion:
“Conclusions: The prevalence of facial side effects is clinically significant in children using NPPV.”
In proposing CPAP as a treatment which is medically equivalent to the TPP treatment, neither Dr. Abadie, nor anyone in Lyon, ever once mentioned to us the significant risks of physical deformities caused by NPPV, risks which are documented by Dr. Fauroux herself.
Dr. Abadie’s CPAP study
In the letter from Dr. Abadie dated 28 April 2017, Dr. Abadie presents ventilation as being medically equivalent to the TPP treatment. In support of her position, she cites the following study, authored by herself, Dr. Fauroux, and other colleagues: “Continuous Positive Airway Pressure for Upper Airway Obstruction in Infants with Pierre Robin Sequence.” This study states that:
“Duration of noninvasive continuous positive airway pressure ranged from 4 weeks to more than 4 months, showing that noninvasive continuous positive airway pressure is necessary only during a critical window of a maximum of 6 months in most patients.”
There are several comments worth making about the above statement.
First of all, this statement does not provide a very clear understanding of what the range of treatment actually was, since the upper end of the range remains undefined: “from 4 weeks to more than 4 months“.
Also, the study appears to draw conclusions based on the evaluation of only 9 patients who actually received ventilatory support:
“In conclusion, during a 1-year period, 44 neonates with Pierre Robin Sequence were evaluated in our reference center before the age of 1 month; seven patients (16 percent) were seen as outpatients and 37 (84 percent) were seen as inpatients. Four patients (9 percent) required a tracheotomy and nine (20 percent) were successfully managed by noninvasive continuous positive airway pressure, with the remaining 31 patients (70 percent) having no significant upper airway obstruction and being managed by prone positioning.”
This relatively small sample size of 9 patients who actually received CPAP makes it doubtful whether this study provides sufficient evidence that CPAP is medically equivalent to the far better documented and more carefully studied TPP treatment.
Furthermore, of the 9 patients in Dr. Abadie’s study who actually received CPAP, Table 1 indicates that 3 of them are “Still on CPAP”. Does this mean that on the date the study was submitted for publication, 3 of the 9 patients who were receiving CPAP were still in fact receiving CPAP, and had already received more than 4 months of CPAP? This might explain the ambiguous date range, “from 4 weeks to more than 4 months“; nevertheless the issue of CPAP treatment duration remains unclear.
Thus the “Continuous Positive Airway Pressure” study uses an ambiguous set of treatment duration data, from a relatively small sample size of 9 patients, to support the following statement:
“…noninvasive continuous positive airway pressure is necessary only during a critical window of a maximum of 6 months in most patients.”
This statement above concerning the maximum 6 month duration period for the typical CPAP treatment is used as the building block of a very important medical conclusion. The medical conclusion is important because it concerns the serious risk that CPAP will cause Pierre Robin Sequence babies to suffer from physical deformities of the face. The conclusion is as follows:
“This relatively short period restricts the potential side effects of long-term noninvasive continuous positive airway pressure such as facial flattening and maxillary retrusion.”
Thus, according to the “Continuous Positive Airway Pressure” study which Dr. Abadie presented to L’Assurance Maladie, the risk that CPAP will cause Pierre Robin Sequence babies to suffer from the physical facial deformity, maxillary retrusion, can be restricted, because the typical Pierre Robin Sequence baby will only need to receive CPAP for a maximum period of 6 months.
This important medical conclusion makes reference to footnote 10. Footnote 10 is a citation containing Dr. Fauroux’s “Facial side effects during noninvasive positive pressure ventilation in children” medical study, which was discussed above. The glaring problem is that Dr. Fauroux’s “Facial side effects” study not only doesn’t support this important medical conclusion, but in fact, Dr. Fauroux’s “Facial side effects” study directly contradicts it. Dr. Fauroux’s “Facial side effects” study makes it very clear that the serious risk of maxillary retrusion – 37% of the patients in her study suffered from it – can NOT be restricted by limiting the duration of treatment. In the “Facial side effects” study, maxillary retrusion was not correlated at all with how many months or how many years the mask was used. Instead, maxillary retrusion was only correlated with the number of hours per day which the mask was worn. According to Dr. Fauroux’s study, the serious risk of maxillary retrusion was triggered when the baby received the ventilation treatment for more than 10 hours per day. Once again, this comes directly from Dr. Fauroux’s “Facial side effects study”:
“A maxillary retrusion was observed in 37% of the patients… In univariate and multivariate analyses only daily use (>10 h per day) was associated with maxillary retrusion (OR 6.3, 95% CI 1.3–29.3, p=0.02)… maxillary retrusion was associated with a daily NPPV use exceeding 10 h per day.”
According to Dr. Fauroux’s “Facial side effects” study, a CPAP mask will not cause a baby to suffer from maxillary retrusion because the baby is wearing the mask for 3 months 6 months or 1 year, but rather because the baby is wearing the CPAP mask for many hours each day. That constant pressure of the CPAP mask on the baby’s face, and on the soft bones of her skull, for 10 hours or more hours each day, is what triggers the risk of maxillary retrusion. As Dr. Abadie told us by telephone, CPAP would be applied “when the baby sleeps”, which is certainly more than 10 hours per day. The point is that according to Dr. Fauroux’s “Facial side effects” study, the serious risk of maxillary retrusion can NOT be eliminated by simply capping the treatment period to “a maximum of 6 months”. The serious risk of maxillary retrusion is triggered when the mask is worn for more than 10 hours a day, even if the treatment period is only a matter of weeks or a few short months.
It is difficult to believe but it is true. To support her claim that CPAP and the TPP treatment are medically equivalent, Dr. Abadie presented L’Assurance Maladie with the “Continuous Positive Airway Pressure” study. This study contains a serious medical conclusion, which minimizes an important health risk – of CPAP causing a physical facial deformity, maxillary retrusion, a deformity of the jaw, in babies already suffering from jaw deformities (retrognathia and micrognathia). In minimizing this important health risk, Dr. Abadie’s study cites Dr. Fauroux’s study… but Dr. Fauroux’s study, which is cited, clearly indicates the opposite.
Did Dr. Abadie, the lead author of “Continuous Positive Airway Pressure”, not read the “Facial side effects” study? Or did she assume that nobody else would read it?
The “Continuous Positive Airway Pressure” study raises additional concerns. The study begins by stating the following:
“Noninvasive continuous positive airway pressure has been shown to be an effective treatment for severe upper airway obstruction in Pierre Robin Sequence.”
This statement above makes reference to footnote 5. Footnote 5 is a citation to the following medical study:
Leboulanger N, Picard A, Soupre V, et al. Physiologic and clinical benefits of noninvasive ventilation in infants with Pierre Robin Sequence. Pediatrics 2010; 126: e1056–e1063.
When one reads this study above, “Physiologic and clinical benefits of noninvasive ventilation in infants with Pierre Robin Sequence”, one finds, once again, that the actual number of patients who received noninvasive respiratory support (“NRS”) is again quite small:
“During the 10-year period, 81 patients with a PRS were treated in our institution. Fifty-four (67%) had an isolated sequence and 27 (33%) had an associated syndrome or malformation. Fifty-one patients (63%) had no or minor respiratory symptoms and were treated with positioning and medical treatment only; 30 patients (37%) had severe respiratory symptoms and required temporary tracheal intubation and positioning (10 patients [12%]), tracheotomy (13 patients [16%]), or NRS (7 patients [9%])… Breathing patterns, respiratory efforts, and gas exchange were analyzed for 7 infants with a PRS during spontaneous breathing and during NRS.”
Apparently, of the 81 PRS patients observed in the “Physiologic and clinical benefits” study, only 7 received NRS.
Thus, it turns out that the “Continuous Positive Airway Pressure” study is based on the use of CPAP for a total of only 9 patients. This is not a large sample size of patients. Therefore, the “Continuous Positive Airway Pressure” study cites the “Physiologic and clinical benefits” study for support. However, the “Physiologic and clinical benefits” study is itself based on only 7 patients who received NRS. This is quite disturbing.
From a salesman one expects a hard sell; a physician however is supposed to present substantial medical evidence, as well as full and honest disclosure of any accompanying health risks. The “Continuous Positive Airway Pressure” study which Dr. Abadie wrote, and which she shared with L’Assurance Maladie, contains neither.
Once again, unless one takes the time to carefully read these CPAP studies, one might incorrectly assume that the “Continuous Positive Airway Pressure” study and the “Physiologic and clinical benefits” study together provide definitive and generalizable conclusions on the efficacy and even safety of providing NRS/CPAP to children suffering from Pierre Robin Sequence. However, they do not. The TPP treatment, on the other hand, rests upon a solid foundation of medical evidence, including peer reviewed medical studies which observed not 9 patients or 7 patients but literally hundreds of patients, spanning over a decade. These were babies whose Pierre Robin Sequence was successfully and safely treated with the TPP technique, requiring hospitalization periods typically ranging in the weeks, not months. These peer reviewed TPP medical studies include:
“Treatment of infants with Syndromic Robin sequence with modified palatal plates: a minimally invasive treatment option”; “Multicenter study on the effectiveness of the pre-epiglottic baton plate for airway obstruction and feeding problems in Robin sequence”; “Initial treatment and early weight gain of children with Robin Sequence in Germany: a prospective epidemiological study”; “Functional treatment of airway obstruction and feeding problems in infants with Robin sequence”; “Treatment of Upper Airway Obstruction and Feeding Problems in Robin-Like Phenotype”; “An Oral Appliance With Velar Extension for Treatment of Obstructive Sleep Apnea in Infants With Pierre Robin Sequence”; and more.
In our S2 application we provided L’Assurance Maladie with hard colour copies of 4 of the abovementioned TPP medical studies. We understand that L’Assurance Maladie is very busy, but did they read any of these studies we sent them?
Yet another issue raised by these studies is that the “Physiologic and clinical benefits” study makes it very clear that the typical duration of NRS treatment is much longer than the “Continuous Positive Airway Pressure” study suggests. The “Continuous Positive Airway Pressure” study states that “…noninvasive continuous positive airway pressure is necessary only during a critical window of a maximum of 6 months in most patients.” However, the “Physiologic and clinical benefits of noninvasive ventilation” study, which the “Continuous Positive Airway Pressure” cites, states that: “All of the patients could be discharged successfully from the hospital with NRS. The mean duration of NRS was 16.7 +/ – 12.2 months.” Table 1 of the “Physiologic and clinical benefits” study confirms this fact, that the typical duration of NRS treatment was far greater than the “maximum of 6 months in most patients” which the “Continuous Positive Airway Pressure” study sets out.
Specifically, the “Physiologic and clinical benefits of noninvasive ventilation”, in Table 1, indicates that the treatment periods for the 7 patients were, respectively: 7 months, 10 months, 13 months, 13 months, 16 months, 27 months, and 39 months. Importantly, these are periods of NRS treatment in which the mask was worn for many hours during each 24 hour period. As the “Physiologic and clinical benefits” study indicates, “Within 2 weeks, NRS was required only during night time sleep and daytime naps.” Thus, the mask was worn “when the baby sleeps”, which is well over 10 hours per day, and the total duration of treatment was quite long. Based on Dr. Fauroux’s, “Facial side effects”, study, all of these children were therefore exposed to the high risk of facial deformities, both global facial flattening, and maxillary retrusion.
Dr. Abadie completely ignores these known risks of facial side effects when informing L’Assurance Maladie that the TPP treatment, and CPAP, are medically equivalent. The TPP treatment has never been shown to produce any risks of any facial side effects or any other physical deformities in any babies. On the contrary, there is medical evidence which suggests that the TPP treatment, by shifting the tongue into a forward position, actually promotes mandibular catch-up growth.
Thus, CPAP creates a high risk of causing additional facial deformities in Pierre Robin Sequence babies who are already suffering from physical deformities of the face, micrognathia and retrognathia. The TPP treatment, on the other hand, offers the possibility of reducing the baby’s existing deformities, micrognathia and retrognathia, by naturally promoting mandibular catch-up growth during a high growth period in the patient’s life.
Prone (stomach) sleeping
For babies suffering from Pierre Robin Sequence in France, the most common treatment appears to consist of “positioning” – placing the baby in a prone (stomach) sleeping position. This is illustrated in both the “Continuous Positive Airway Pressure” study, as well as in the “Physiologic and clinical benefits” study. In the “Physiologic and clinical benefits” study, it appears that 75% of the patients were treated with positioning, or some combination of positioning and other strategies (63% “were treated with positioning and medical treatment only”; an additional 12% “required temporary tracheal intubation and positioning”). In the “Continuous Positive Airway Pressure” study, 70% of the Pierre Robin Sequence babies were “managed by prone positioning” alone.
In Lyon, Lysiane was “treated with positioning”. However, this treatment proved inadequate. From the moment of Lysiane’s birth, we as parents were in the Intensive Care unit every single day, spending time with Lysiane. Lysiane was placed on her stomach to sleep, and due to her breathing difficulties, she was also frequently placed on her stomach even when she was awake.
In spite of this treatment by prone (stomach) positioning, we continued to see Lysiane not only struggling to breathe, but also desaturating, time and time again. We knew that Lysiane was desaturating because the monitoring equipment which Lysiane was connected to would flash red lights, and sound urgent alarms. This was a regular event, and since we as parents knew that these repeated episodes of hypoxemia were putting Lysiane at risk of brain damage, these desaturation events were horrifying.
The failure of “positioning” to resolve Lysiane’s breathing difficulties led the doctors in Lyon to combine “positioning” with an additional treatment strategy, ventilation assistance. The medical report (“RESUME DE SEJOUR”) prepared by Lysiane’s attending neonatologist, Dr. Nathalie Mory Thomas, indicates that high-flow nasal cannula ventilation was applied:
“as a result respiratory fatigue with signs of struggle and hypercapnia” [abnormally high levels of carbon dioxide in the blood]
Original text, in French:
“sur fatigue respiratoire avec signes de lutte et hypercapnie”
However, even after the doctors put Lysiane on the high-flow nasal cannula, the prone (stomach) positioning continued; prone positioning was used in conjunction with ventilation. Below, Lysiane receiving ventilation assistance (high-flow nasal cannula), and also placed in a prone sleeping position:
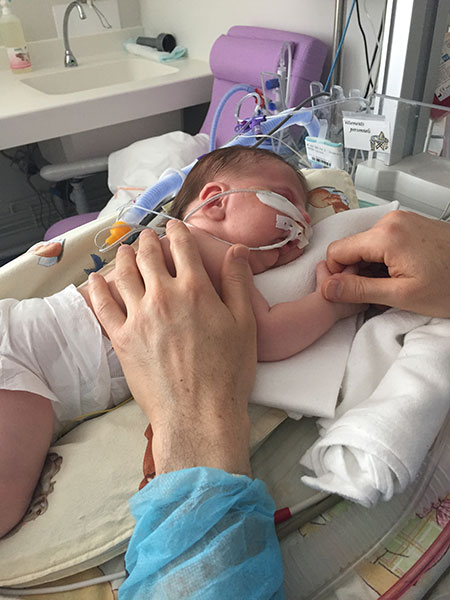
Ventilation assistance combined with prone (stomach) sleeping position:
Prone (stomach) sleeping is something that we as parents were always deeply uncomfortable with. During the prenatal period, we had made numerous trips to the hospital for standard checkups and medical ultrasound examinations. In all of the different waiting rooms we sat in, during all of these numerous prenatal appointments, we always saw this same sign, posted in each of the waiting rooms:

What this poster says, in English, is:
“Even if everything in this room looks perfect… a baby sleeping on her stomach runs a mortal risk. Do not make this mistake! Your baby should ALWAYS sleep on her back.”
Having seen these posters, and having heard about Sudden Infant Death Syndrome (“SIDS”), we as parents were highly uncomfortable with the idea of our child Lysiane sleeping on her stomach. We read medical studies which made it very clear that prone (stomach) sleeping substantially increases the risk of SIDS, anywhere from five times, to as much as 13 times [R G Carpenter, L M Irgens, et. al. Sudden unexplained infant death in 20 regions in Europe: case control study. The Lancet 2004: 363: 185-191, showing that prone sleeping increased the risk of SIDS 13 times; Table 2, “Prevalence of multivariately significant potential risk factors for SIDS in cases and controls”, indicates that the Risk factor, “Prone vs supine”, produced a Multivariate OR (95% CI) of 13·1 (8·51–20·2)].
In spite of these known risks, Lysiane was attached to monitors, and placed on her stomach to sleep. We were told that the purpose of stomach sleeping for a child suffering from Pierre Robin Sequence is that with stomach sleeping, the baby’s face is pointing down; this causes the baby’s tongue to fall forward in her mouth, and thus out of the way of her throat. With the tongue falling forward in the mouth, and out of the way of the throat, the baby’s upper airway obstruction is temporarily relieved.
Although this stomach sleeping was done with monitoring, we as parents still couldn’t help but think of this situation as a kind of medical schizophrenia. The same medical establishment which was issuing dire warnings concerning stomach sleeping, was now telling us that we should accept the risks, and place our baby to sleep on her stomach – but to not worry, because everything was going to be OK.
Imagine for a moment traveling in a strange and distant land. You and your travel guide come before a field. The field is enclosed by a barbed wire fence, and is marked with a huge sign, warning you that the field ahead is a deadly minefield. The sign explains that if you walk through this minefield, you will be exposing yourself to the grave risk of sudden death. “Don’t worry”, your guide tells you – “I’ve got this trustworthy mine detector over here. This mine detector is going to detect all the mines in this minefield. Trust me, you don’t need to worry about a thing; we’re going to be completely safe.”
What would you do? Would you cross this minefield? Would you knowingly expose yourself to the grave risk of sudden death?
Wouldn’t it make more sense to simply walk around the minefield, and avoid the risk altogether?
Now for a moment imagine that it’s not your life which is at stake. Instead, it’s the life of your baby child – that is, your guide is not telling you to walk through the treacherous minefield – rather, your guide is telling you to send your baby through that minefield, all alone. Your job is to just stand there quietly, and watch.
This is what it was like for us, as parents, to stand there for weeks and weeks and weeks on end, while the doctors knowingly exposed our child to the substantially increased risk of SIDS, by placing her in a prone (stomach) sleeping position.
We as parents strongly believe that the proven dangers of prone sleeping are so great that prone sleeping should only be prescribed if it is absolutely necessary – i.e., when there is no alternative treatment available which would allow our child to avoid the SIDS minefield altogether.
The TPP treatment achieves this goal. The TPP treatment corrects the underlying problem, Pierre Robin Sequence’s upper airway obstruction, in both the prone (stomach) sleeping position, as well as in the supine (back) sleeping position. Thus, with the TPP treatment, the baby never has to sleep in the prone sleeping position again.
A rare disease requires a particular concentration of expertise
Lysiane, who suffers from a rare disease, is a patient “with a medical condition requiring a particular concentration of expertise in medical domains where expertise is rare” (the 2011 Directive, Article 12). Regarding rare diseases, the 2011 Directive, Article 13(a), names Orphanet, a database, to provide information about each rare disease, and to identify the Centres of Expertise in Europe for each rare disease. When looking up the Pierre Robin Sequence rare disease on Orphanet, one finds the following:
“Prenatal diagnosis is possible if the retrognathism is detected at ultrasound. An excess of amniotic fluid is a good diagnostic indicator.
Expert reviewer(s): Pr Véronique ABADIE – Last update: March 2006″
Found here: http://tinyurl.com/pierre-robin-orphanet
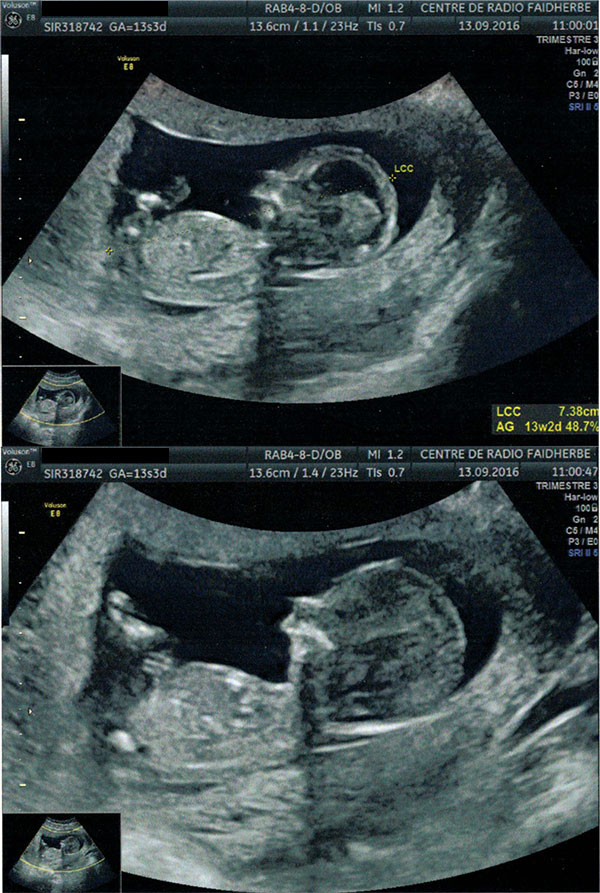
We did have a prenatal ultrasound, in fact several of them – and our prenatal ultrasounds did indicate that our child suffered from retrognathia.
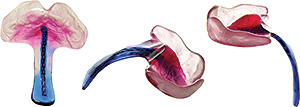
First, an illustration from the medical study, “Prenatal Identification of Pierre Robin Sequence”. Below are two ultrasound images. The ultrasound image to the left shows a normal foetal facial profile. The ultrasound image to the right shows the foetal profile of a baby with Pierre Robin Sequence, suffering from retrognathia and micrognathia.
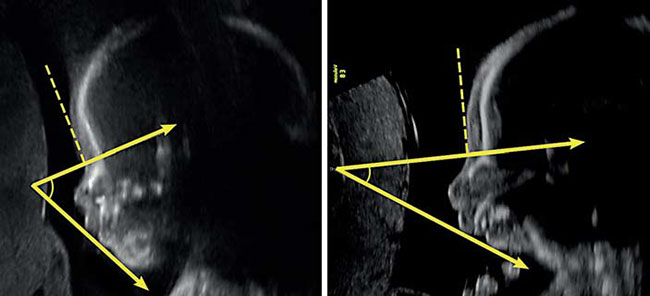
[Kaufman, Matthew G; Cassady, Christopher I; Hyman, Charles H; Lee, Wesley; Watcha, Mehernoor F; Hippard, Helena K; Olutoye, Olutoyin A; Khechoyan, David Y; Monson, Laura A; Buchanan, Edward P. Prenatal Identification of Pierre Robin Sequence: A Review of the Literature and Look towards the Future. Fetal Diagnosis and Therapy 2016; 39: 81-9.]
What follows are actual prenatal ultrasound images of Lysiane showing retrognathia, and indicating Pierre Robin Sequence – critical warning signs which were all overlooked.
13 September 2016 ultrasound, Paris:
21 November 2016 ultrasound, Paris:
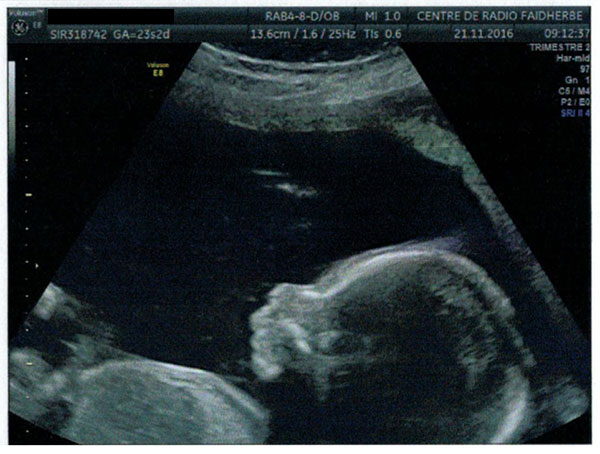
27 January 2017 ultrasound, Lyon:
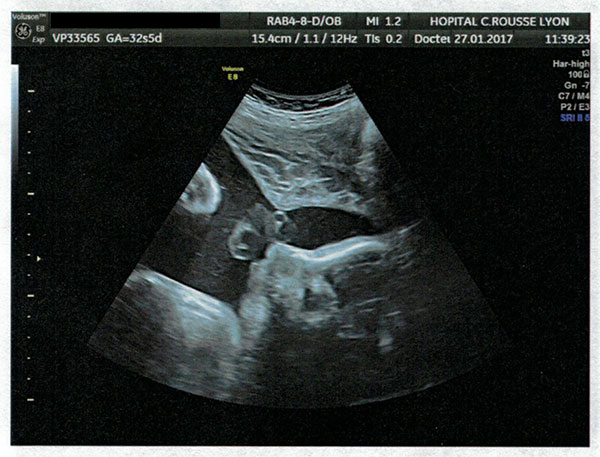
27 January 2017 ultrasound, Lyon:
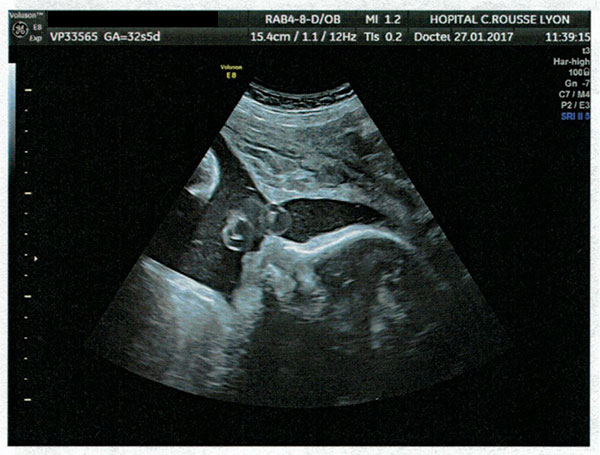
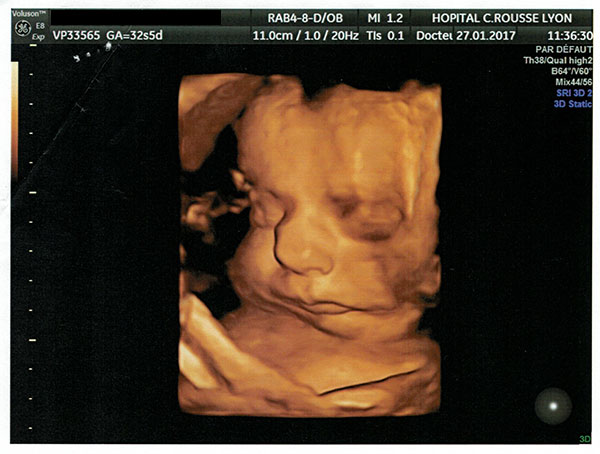
In addition to the ultrasound images showing retrognathia, there was also a second entire set of warning signs, an excess of amniotic fluid, which as Orphanet explains is “a good diagnostic indicator” of Pierre Robin Sequence. During the pregnancy, an excess of amniotic fluid was observed. This excess of amniotic fluid was specifically noted in multiple echographie (ultrasound) reports, including the echographies performed on:
- 18 January (“croissance au 50°P MAIS EXCES DE LIQUIDE AMNIOTIQUE INDEX 22”);
- 27 January (“Le liquide amniotique est augmenté avec excès de LA. Index du liquide amniotique: Plus grande citerne 62,0 cm, Index LA: 21,2 cm.”); and
- 14 February (“Le liquide amniotique est augmenté avec excès de LA. Index du liquide amniotique: Plus grande citerne 6,2 cm [sic], Index LA: 19,3… Conclusion: Persistance de l’excès de LA, pas des autres signes d’appel échographique”).
In addition to being noted in several echographie reports, this excess of amniotic fluid was discussed several times during █████’s prenatal examinations. As a result, █████ was ordered to cancel a planned trip, remain at home, and limit her movements to an absolute minimum.
Thus, in Lyon, two critical sets of warning signs indicating that Lysiane had Pierre Robin Sequence were overlooked. This failure in Lyon to identify two critical warning signs pointing to Pierre Robin Sequence had consequences at birth.
The medical file from Lyon indicates that one minute after birth, Lysiane’s Apgar’s score was 1. The next Apgar figure was still critical: it was 4. The question is, what happened during this important initial interval? The summary that was prepared by the hospital in Lyon, for the German hospital, indicates the following:
“Initial treatment:
Resuscitation: Aspiration: pharyngeal. VAM: for 3 minutes
Intubation: at 29 minutes of life. Tube: number 3.5. Tube length: 11cm.
Immediate postnatal development:
14h11: treatment by the midwives… Call for JM Labaune in the face of difficulties providing ventilation, attempt to place oropharyngeal cannula/Guedel by midwives. Failure.
14:14: arrival Dr. JM Labaune: Pierre Robin Sequence. Placement of laryngeal mask. Poor adaptation despite laryngeal mask: intubation tube number 4.”
Original text, in French:
“Prise en charge initiale :
Réanimation : Aspiration : pharyngée. VAM : pendant 3 minutes
Intubation : à 29 minutes de vie. Sonde : numéro 3,5. Repère Sonde : 11cm.
Evolution postnatale immédiate :
14h11 : prise en charge par les sages-femmes…Appel JM Labaune devant difficultés d’efficacité de la ventilation essai de pose de canule de Mayo par SF. Echec.
14h14 : arrivée Dr JM Labaune : Séquence de Pierre Robin. Pose masque laryngé. Mauvaise adaptation malgré masque laryngé : intubation sonde no 4.”
What the above suggests is that at birth, Lysiane was not breathing. According to the medical report (“RESUME DE SEJOUR”) prepared by Lysiane’s attending neonatologist, Dr. Nathalie Mory Thomas, at birth Lysiane suffered from respiratory distress as a result of an inhalation of clear amniotic fluid, and obstruction caused by Pierre Robin Sequence:
“ILAC [Inhalation de Liquid Amniotique Clair] + obstruction from Pierre Robin Sequence”
Original text, in French:
“ILAC + obstructive sur séquence de Pierre Robin”
More important however are the actions which were carried out: three critical minutes were devoted to the application of “VAM”, which probably refers to manual ventilation assistance of some kind. This did not work, so a doctor was called in. The reason this is important is that it is difficult to provide ventilation assistance to a baby with Pierre Robin Sequence, due to the baby’s very specific anatomical defects. Lysiane suffers from micrognathia, the incomplete development of her lower jaw, and retrognathia, the abnormal positioning of her lower jaw to the posterior. In informal language, micrognathia means that the baby has a very small lower jaw and a small chin, while retrognathia resembles a severe overbite. With these anatomical defects, medical instruments that work on a face with normal facial anatomy will fail, because the lack of a chin makes it difficult or impossible to create a seal on the face using a standard facial mask.
If the hospital in Lyon had been able to recognize the two warning signs of Pierre Robin Sequence – the ultrasound images which showed that Lysiane had retrognathia, and the excess amniotic fluid – then the hospital could have anticipated a difficult birth, the birth of a baby with Pierre Robin Sequence. Instead of spending three valuable minutes trying to apply VAM, they could have gone directly to the laryngeal mask and intubation, knowing in advance that the anatomical defects of the baby would make ventilation difficult or impossible using the standard tools and procedures. The birth of Lysiane illustrates why it is so important for a patient with a rare disease to receive treatment from caregivers who have highly specialised knowledge about the rare disease.
According to Orphanet:
Isolated Pierre-Robin sequence (without any other associated malformation) occurs in about 50% of cases… In one half [the other half] of the cases, Pierre-Robin sequence occurs as part of a complex malformation syndrome.
Found here: http://tinyurl.com/pierre-robin-orphanet
Since about half of all patients with Pierre Robin Sequence also suffer from another associated condition, physicians attending to a patient with Pierre Robin Sequence must be vigilant to check for signs of the other associated conditions. The medical condition that is most often associated with Pierre Robin Sequence is a condition known as Stickler’s Syndrome, which is a genetic disease affecting connective tissue.
During a meeting which took place on 7 April 2017 we discussed our concerns with the neonatologist in charge of Lysiane’s care. We raised the issue of associated conditions. We were surprised when the neonatal physician asked us, “like what?” We assumed that she would know the conditions most frequently associated with Pierre Robin Sequence, and that she would explain these associated conditions to us. █████ said, “for example, Stickler’s Syndrome.” The doctor looked at us, and asked █████ to repeat it. “I do not know that,” said the doctor. She turned to the medical student sitting next to her; the medical student did not know either. The medical student literally went to her computer, did a Google search for Stickler Syndrome, and read aloud what was on the web page. The doctor then looked at us, and admitted, “I do not know Stickler’s Syndrome.” We were stupefied.
If the doctor treating our daughter doesn’t even know what she should be looking for, how is she ever going to find it? Once again, this proves the point that a patient with a rare disease requires “a particular concentration of expertise in medical domains where expertise is rare” (the 2011 Directive, Article 12).
The TPP treatment: a step forward in the treatment of this rare disease
Dr. Brigitte Fauroux, from the Pediatric Noninvasive Ventilation and Sleep Unit of Necker Hospital in Paris, is an expert in the use of ventilation. Regarding ventilation, Dr. Fauroux has written that:
“The better comfort of this noninvasive technique [ventilation], as compared with a tracheostomy, is likely to translate into a better quality of life, not only for the patients but also for their families.”
One can draw at least two significant conclusions from Dr. Fauroux’s noteworthy remark on ventilation. The first is that all things being equal, a noninvasive technique (ventilation) is preferable to an invasive technique (tracheostomy).
The second lesson is that quality of life – for patients, and for the families of patients – is a critical issue to take into account when choosing a medical treatment. This principle is even more important in the context of a rare disease. Patients should take quality of life into account, the families of patients should take quality of life into account, and doctors themselves should take quality of life into account, when they recommend possible treatment options. If we, as parents of a child suffering from Pierre Robin Sequence, were forced to choose between tracheostomy and ventilation, we would absolutely choose ventilation; in fact we would prefer ventilation over any surgical intervention, if we had a choice in the matter. At the same time, we believe that numerous peer reviewed medical studies have definitively proven, over more than a decade, that the TPP treatment represents the next big step forward in the treatment of Pierre Robin Sequence. When one honestly compares the tiny and portable TPP device, with the ventilation equipment and tubes and extended medical supervision required for ventilation, then it becomes very easy to extend Professor Fauroux’s logic to the TPP treatment; the better comfort of the TPP device, as compared with ventilation, is likely to translate into a better quality of life, not only for the patients, but also for their families.
| The Evolution of treatments for Pierre Robin sequence | ||||
|
Tracheotomy
|
. .
|
CPAP
|
. .
|
TPP treatment
|
Although the effectiveness of the TPP treatment has been medically proven for over ten years, and represents a major step forward in the treatment of this rare disease, Pierre Robin Sequence, we believe that Lysiane, our daughter, is the first French baby to ever receive this proven treatment. This is surprising, considering that France is a country of 65 million people. Also, France is one of the richest countries in the entire EU, and can afford to support its citizens in obtaining cross-border healthcare, especially the relatively few French citizens suffering from a rare disease. Finally, France and Germany are next door neighbours. Why then have babies in France never been transferred to Germany for the proven TPP treatment, when babies from Austria, Hungary, the Czech Republic and other EU Member States have been transferred to Germany – and even babies from as far away as Russia have been transferred to Germany to receive the TPP treatment?
Also: if the effectiveness of the TPP treatment has been medically proven for more than 10 years, then why isn’t the TPP treatment available in France? In particular, why isn’t the TPP treatment being offered at the Pierre Robin Reference Centre in Paris? The policy of the Pierre Robin Reference Centre is that ventilation is just as effective as the TPP treatment, and therefore there is no need for France to adopt the TPP treatment. Imagine if the French Minister of Information Technology prevented the adoption of e-mail in France, based on the argument that people in France do not need e-mail – they can continue using fax machines instead. The problem is that this type of attitude can have very serious consequences in the realm of human health. When discussing treatments for a rare disease, the refusal to adopt medically proven innovations can subject patients – including innocent newborn babies – to physical harm, suffering, and even death.
In all fairness, there exists no medical or legal obligation for the physicians at the Pierre Robin Reference Centre in Paris to learn the TPP treatment, and offer it to Pierre Robin Sequence patients in France. The 2011 Directive, on the other hand, does indeed create obligations; it obliges all EU Member States, including France, to respect an EU citizen’s right to choose the best medical treatment possible, particularly in the context of a rare disease – “even for diagnosis and treatments which are not available in the Member State of affiliation” (the 2011 Directive, Article 13, “Rare diseases”). If the Pierre Robin Reference Centre in Paris wishes to intentionally disregard the TPP treatment for Pierre Robin Sequence babies in France, it may do so – but it cannot at the same time obstruct French babies from leaving France, and receiving this medically proven rare disease treatment where it is available, in another EU Member State.
Click here to proceed to Part 3, the Legal Analysis.
You can also view the letter as a PDF, which you can download and read offline: