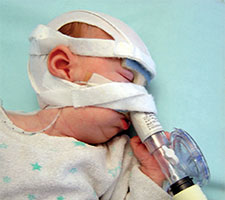
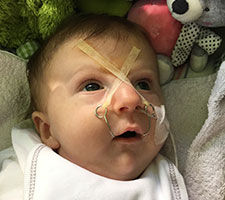
Lysiane
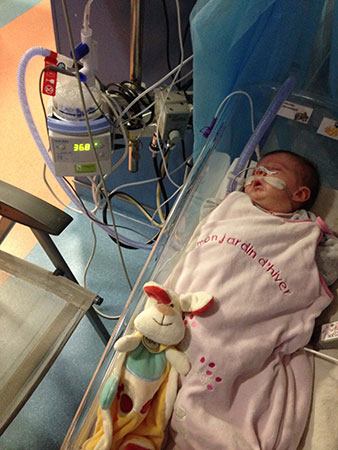
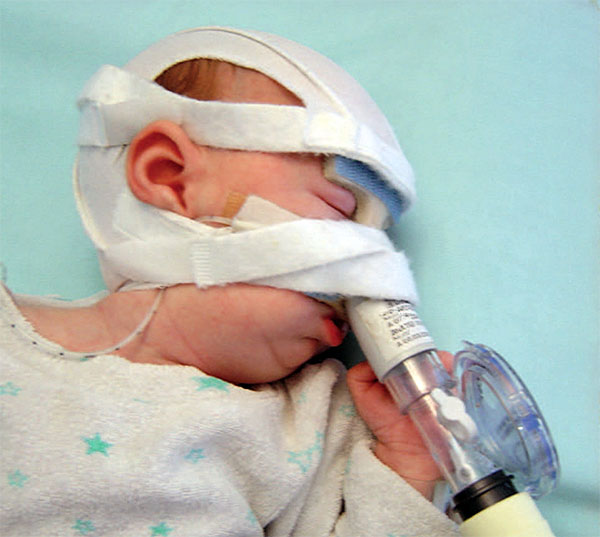
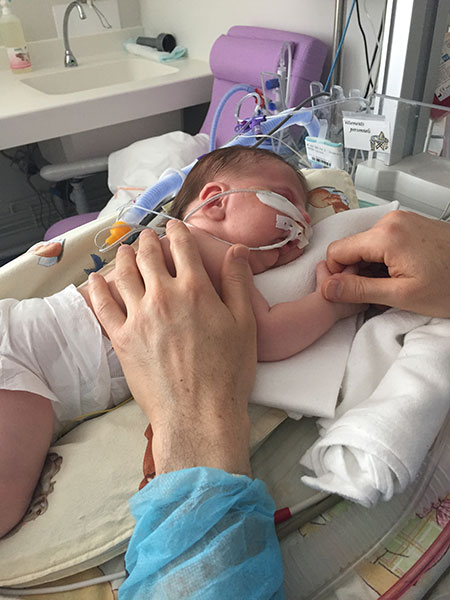
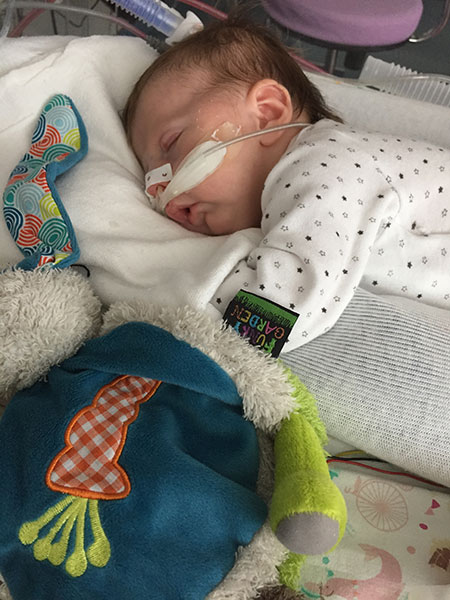
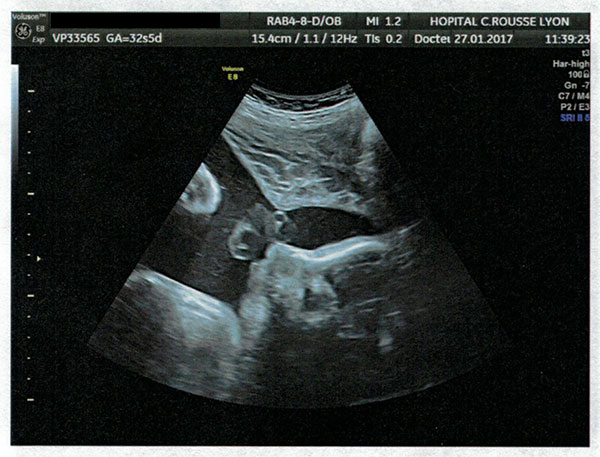
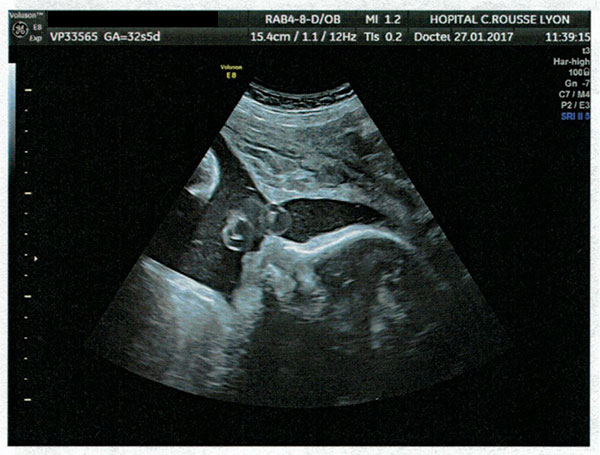
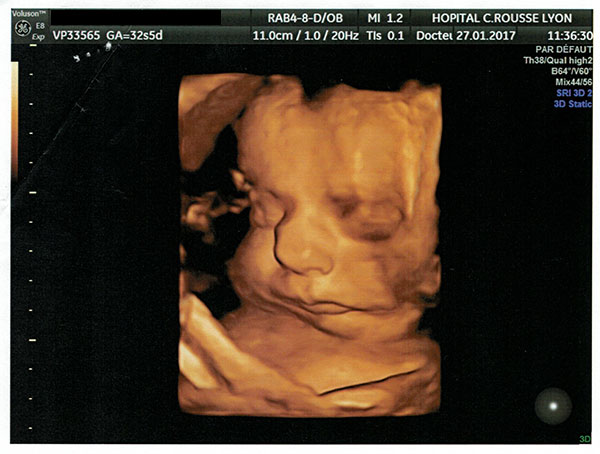
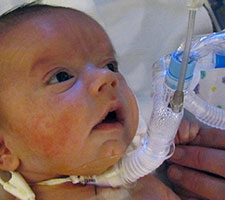
On 29 March 2017 my partner gave birth to a baby girl, Lysiane, who was diagnosed with a rare disease called Pierre Robin Sequence. Pierre Robin Sequence, which is also called Pierre Robin Syndrome, is a rare disease which strikes only approximately 1 out of 10,000 babies. Pierre Robin Sequence is associated with potentially life threatening breathing problems and eating difficulties; babies with this rare disease face many risks, including oxygen deprivation, brain damage, failure to thrive, and death.
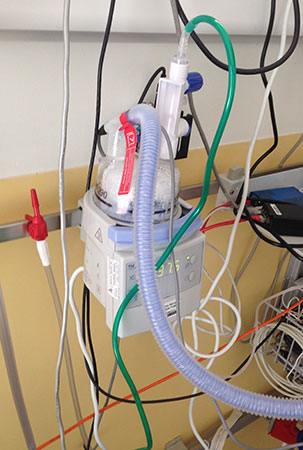
Our daughter Lysiane suffered a dangerous, difficult birth. She never came home from the French hospital. She was attached to a ventilator machine in the intensive care unit, and she remained in that intensive care unit, in the same French hospital where she was born, for five straight weeks – with no scheduled date of release.
A breakthrough treatment for Pierre Robin Sequence, developed in the EU
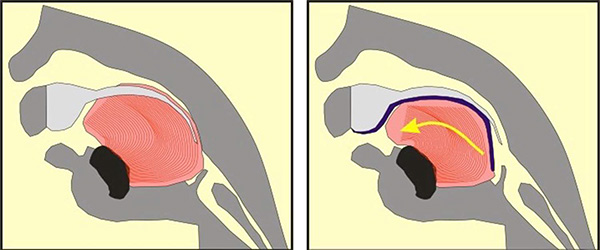
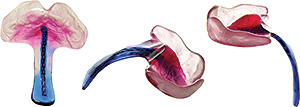
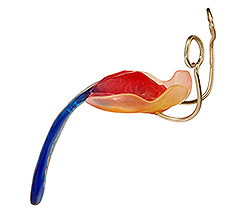
Doctors in Germany have spent over 20 years developing a highly specialised, medically proven and cost-effective treatment for Pierre Robin Sequence. The treatment is called the Tübingen Palatal Plate – the “TPP” treatment. The TPP is a safe, non-surgical, oral medical device which resolves the baby’s torturous breathing difficulties, liberates the baby from the ventilator machine, and eliminates the need for invasive surgical procedures. The TPP represents a dramatic breakthrough in the treatment of Pierre Robin Sequence.
As you can imagine, we as parents felt incredibly lucky to learn that this breakthrough Pierre Robin Sequence treatment was developed here in the EU, and in fact that it was available just next door, in Germany. This, we thought, was one of the special advantages of living in the European Union: collaboration and pooling of knowledge between EU Member States, allowing all European citizens, especially babies suffering from a rare disease, to benefit from Europe’s great medical innovations.
In accordance with EU law, we, Lysiane’s parents, both EU citizens, submitted an application to France’s national health insurance fund, “L’Assurance Maladie”. In our application we requested official authorisation – what is called an “S2” form – to permit our daughter Lysiane, herself a French citizen, to receive the highly specialised, medically proven and cost-effective TPP treatment in Germany…
The French government rejects our request for the TPP treatment in Germany
The French government rejected our daughter Lysiane’s S2 application to receive the TPP treatment in Germany.
We believe that the French government’s rejection of our S2 request for this highly specialised and medically proven German healthcare treatment was a shameful violation of European law. Why? Because based on the EU’s 2011 Cross-border Healthcare Directive, Article 13, “Rare diseases”, EU Member States are legally required to respect an EU citizen’s right to choose the best medical treatment available in the EU, especially in the context of a rare disease, “even for diagnosis and treatments which are not available in the Member State of affiliation”. It is a confirmed fact that the TPP treatment in Germany is not yet available in France. Furthermore, there is no treatment which is available in France which is equally effective as the TPP treatment.
Our allies
We are not alone in considering the French government’s rejection of our request for the highly specialised TPP rare disease treatment in Germany to be a violation of EU law; our growing list of allies includes:
- The European Commission’s SOLVIT Network. After carefully analysing our case, the European Commission’s SOLVIT Network formally concluded that CPAM, in rejecting our daughter Lysiane’s request for cross-border healthcare, violated EU law (SOLVIT Case Number 2569/17/DE). SOLVIT’s conclusion was that Lysiane qualified for authorisation for cross-border healthcare under both the 2011 Cross-border Healthcare Directive, as well as under Regulation 883.
- A senior Member of European Parliament from the 8th, 7th, 6th, 5th and 4th Parliamentary Terms, Ms. Françoise Grossetête. Ms. Grossetête has extensive experience working with the EU’s 2011 Cross-border Healthcare Directive; she was the European Parliament’s Special Rapporteur for this legislation. Ms. Grossetête knows this law, and she too knows it was violated; she sent a formal letter of support directly to France’s Minister of Health, Madame Agnès Buzyn, making it clear that this situation is “unacceptable”, and that the rejection was legally unfounded.
- EURORDIS, the largest rare disease patient organization in the EU (Yann Le Cam, CEO, the European Organisation for Rare Diseases). EURORDIS sent a highly detailed letter of support to CPAM, explaining why they too believe the rejection violated EU law. EURORDIS has pledged to accompany us, Lysiane’s parents, in court, all the way up to the Court of Justice of the European Union.
- The European Patients’ Forum, the largest patient advocacy umbrella organization in the EU. The European Patients’ Forum was a key stakeholder in the drafting of the 2011 Cross-border Healthcare Directive; they too know this law. The European Patients’ Forum sent a formal letter of support directly to the French President, Emmanuel Macron. In their letter they state that this situation is “shocking”, and that CPAM’s rejection was legally unfounded.
- Rare Diseases Denmark, a leading rare disease advocacy group from Scandinavia, and a highly respected voice in the international rare disease movement. In their letter of support (Lene Jensen, CEO, Rare Diseases Denmark; former member of EUCERD; former member of Danish Parliament), they point out that the case of Lysiane “is a devastating example of the struggle that many rare disease patients and families have faced, and continue to face, right here in Europe.”
- Pro Rare Austria, an Austrian umbrella organization for rare disease patient advocacy groups. In their letter to CPAM they ask the Director, Nicolas Revel, to reverse CPAM’s unfounded rejection of Lysiane’s right to a safe and medically proven rare disease treatment in Germany.
- The Member of Parliament representing our district in France, Mr. Bruno Bonnell, of the Assemblée Nationale (France’s Parliament). MP Bonnell is an active defender of patients’ rights and people with disabilities.
- John Bowis, former Member of European Parliament and Special Rapporteur for the 2011 Cross-border Healthcare Directive
- Member of European Parliament Petra De Sutter, MD, PhD; Chair, European Parliament Committee on the Internal Market and Consumer Protection; Physician
- Member of European Parliament Tilly Metz, Member, European Parliament Committee on the Environment and Public Health
- Member of European Parliament Olivier Chastel, Vice-Chair, European Parliament Committee on Budgets
- Member of European Parliament Frédérique Ries, Member for over 20 years now in the European Parliament Committee on the Environment and Public Health, Member of the European Parliament Committee on Petitions, Substitute, the European Parliament Subcommittee on Human Rights, Vice-Chair, Renew Europe Group
- Member of European Parliament Evelyne Gebhardt, Member, European Parliament Committee on the Internal Market and Consumer Protection
- Professor Andre den Exter, Jean Monnet Chair of European Union Health Law, Erasmus University, Rotterdam
- Professor Francis Kessler, Social Security Law, Comparative Law and European Social Law, Sorbonne Université Paris 1, Paris
- Professor Dominic Wilkinson, Medical Ethics, Oxford University, Oxford, and Physician specialising in newborn intensive care; Editor, The Journal of Medical Ethics
- Professor Frans Pennings, Labour Law and Social Security Law, University of Utrecht, Utrecht
- Professor Victor G. Rodwin, Health Policy and Management, New York University, New York City
- Cor Oosterwijk, PhD, Director, VSOP (Vereniging Samenwerkende Ouder en Patiëntenorganisaties), The Dutch Patient Alliance for Rare and Genetic Diseases
- Durhane Wong-Rieger, PhD, President & CEO, CORD, the Canadian Organization for Rare Disorders
- Justina Januševičienė, PhD of Public Law, Head of Health Care Innovation Development Center at Lithuanian University of Health Sciences
- Nick Sireau, PhD, Chairman and CEO, AKU Society
- Willy Palm, Senior Adviser, European Observatory on Health Systems and Policies; author, “Everything you always wanted to know about European Union health policies but were afraid to ask”.
- Ing. Mgr. Pavla Mašková, PhD, Česká asociace pro vzácná onemocnění, The Czech Association for Patients with Rare Diseases
- Jasmin Barman-Aksözen, PhD, Vice-president, International Porphyria Patient Network (IPPN)
- Yvonne Milne, Founder, Rett UK, Board Member, Rett Syndrome Europe, European Reference Network (ERN) Patient Advocacy Group Representative, MBE for services to health
- Heather C. Etchevers, PhD; Research Scientist, INSERM
- Holm Graessner, PhD, MBA, FEAN; Coordinator, European Reference Network for Rare Neurological Diseases; Managing Director, Centre for Rare Diseases Tübingen
- Lieven Bauwens, rare disease patient advocate (Spina Bifida and Hydrocephalus); advocate for the rights of persons with disabilities
- Irena Žnidar, PhD, Director, International Gaucher Alliance (IGA)
- Alastair Kent, Policy Expert on rare and genetic diseases; OBE for services to healthcare; Fellow of the Royal Society of Arts; EURORDIS Lifetime Achievement Award
- Professor Scott L. Greer, Health Management and Policy, Global Public Health, and Political Science, the University of Michigan, Ann Arbor; Senior Expert Advisor on Health Governance for the European Observatory on Health Systems and Policies; author, “Everything you always wanted to know about European Union health policies but were afraid to ask”
- Professor Olivier De Schutter, International Human Rights law, European Union law, and legal theory, the University of Louvain, Louvain-la-Neuve, as well as at Sciences Po in Paris; Member, the UN Committee on Economic, Social and Cultural Rights; author, “Infringement Proceedings as a Tool for the Enforcement of Fundamental Rights in the European Union”
Every single one of these people and organisations agree that whatever the French government’s real reason was for rejecting Lysiane’s S2 application to obtain the highly specialised, medically proven and cost-effective TPP treatment in Germany, the rejection violated European law.
We, Lysiane’s parents, are formally appealing the rejection. We are continuing our fight to obtain the S2 cross-border healthcare authorisation form which our daughter Lysiane was wrongfully denied.
Why our appeal is important
Our struggle to exercise our legally guaranteed right to access cross-border healthcare in the EU is not just about our baby Lysiane. And it is not just about us. And it is not just about other babies who are suffering from this particular rare disease, Pierre Robin Sequence. It is broader than that.
This case raises an issue of wider principle which matters to all EU citizens, and which affects their daily lives: if a newborn baby suffering from a rare disease, immobilized in an intensive care unit and connected to a ventilator machine, doesn’t have the right to obtain a highly specialised, medically proven and cost-effective treatment for her rare disease in another EU Member State – then who does have the right to obtain cross-border medical care?
We, Lysiane’s parents, both EU citizens, appeal to France’s President, President Macron, and to the leadership of the European Union in Brussels, to correct this glaring injustice.
Letter to President Macron
23 February 2018
Monsieur le Président de la République Française
Palais de l’Elysée
55 Rue du Faubourg Saint-Honoré
75008 Paris
France
Re: Lysiane Pakter – French baby with a rare disease
If a newborn French baby suffering from a rare disease, immobilized in an intensive care unit and connected to a ventilator machine, doesn’t have the right to obtain a highly specialised, medically proven and cost-effective treatment for her rare disease in another EU Member State – then who in France does have the right to obtain cross-border medical care?
Dear President Macron,
My partner and I are writing to you to urgently request your intervention in a matter involving our baby, Lysiane, who suffers from a rare disease, Pierre Robin Sequence, and our continuing struggle to obtain an S2, L’Assurance Maladie’s administrative approval, for a highly specialised, medically proven and cost-effective treatment, the “TPP” treatment in Germany, for her rare disease. Our intervention request is for your assistance obtaining this S2, which we were wrongfully denied.
France’s Reference Centre for this rare disease confirmed that the TPP treatment is not available in France, but that it is available in fellow EU Member State, Germany. Nevertheless, L’Assurance Maladie rejected our request for this treatment, and denied us the S2. In spite of our efforts, L’Assurance Maladie continues to maintain its unfounded rejection; this is why we have finally decided to make this heartfelt appeal to you. We as parents want this administrative ordeal with L’Assurance Maladie to finally be resolved, so that we can focus our energy where it belongs: on our baby Lysiane, who suffers from a rare disease.
L’Assurance Maladie’s rejection of our request for this highly specialised rare disease treatment violates the 2011/24/EU Directive on Cross-border Healthcare, Regulation (EC) No 883/2004 on Social Security Systems, and the fundamental principle of the freedom to provide and receive services under Article 56 of the Treaty on the Functioning of the European Union. It infringes upon the EU’s four fundamental freedoms – the free movement of goods, services, people and capital; it undermines a key EU policy objective at the heart of the European project – the effort to create a single EU market; and it cannot possibly be justified as “necessary and proportionate” on any public policy grounds. We have a growing list of individuals and international organizations who have carefully analysed our case, and who openly agree with our position. Attached please find several letters of support, beginning on page 4. Our allies include:
- the European Commission’s SOLVIT network, which agrees with our legal position, Case Number 2569/17/DE;
- the Member of Parliament representing our district in France, Mr. Bruno Bonnell, of the Assemblée Nationale; Mr. Bonnell is an active defender of patients’ rights, and people with disabilities; he is supporting us in our appeal;
- EURORDIS, the largest rare disease patient organization in the EU; EURORDIS has pledged to accompany us in court, all the way up to the European Court of Justice; EURORDIS has sent a formal letter of support to L’Assurance Maladie;
- a Member of European Parliament, Ms. Françoise Grossetête, who has extensive experience working with the 2011/24/EU Directive on Cross-border Healthcare; Ms. Grossetête has sent a formal letter of support to France’s Minister of Health, Ms. Agnès Buzyn;
- the European Patients’ Forum, the largest patient advocacy umbrella organization in the EU, and a key stakeholder in the drafting of the 2011 Directive; the European Patients’ Forum has sent a formal letter of support directly to you, President Macron;
- an international network of law professors who specialise in EU cross-border healthcare and social security law, who agree that L’Assurance Maladie’s rejection was unfounded.
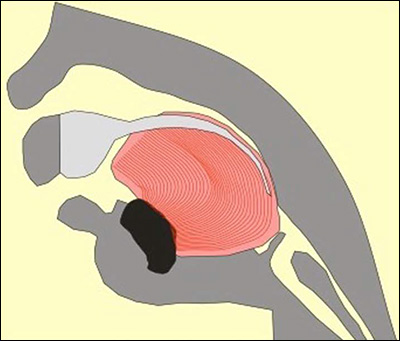
As we explain in this document, the treatment which is used in France to treat our baby’s rare disease, Pierre Robin Sequence, consists of keeping the baby in a hospital, attached to a ventilator machine. Since ventilation assistance generally requires long term hospitalisation, the French treatment creates colossal healthcare costs, and it uses up precious space in neonatal intensive care units. Germany’s highly specialised TPP treatment, on the other hand, is medically proven to resolve the upper airway obstruction associated with this rare disease. The TPP treatment liberates the baby from the ventilator machine without surgery, and without long term hospitalization, making it a cost-effective medical breakthrough in the treatment of this rare disease.
Thus, not only does the French treatment have the disadvantage of keeping the baby attached to a ventilator machine, substantially reducing mobility, and quality of life – but in addition to this, it doesn’t even make financial sense. For L’Assurance Maladie in France to deliberately obstruct a French baby’s access to the medically proven German treatment, which dramatically improves the baby’s ability to breathe, and quality of life – and which reduces healthcare costs, by eliminating the need for long term hospitalisation – is as irrational as refusing to adopt email, and stubbornly insisting on using expensive and cumbersome fax machines instead.
Since the lives and suffering of newborn babies is at stake, L’Assurance Maladie’s rejection doesn’t just defy logic; it is also unconscionable. No ethical doctor or civil servant should force a newborn baby suffering from a rare disease to endure long term intensive care, with all of the burdens this entails, when fellow doctors in the EU Member State just next door have successfully developed a cost-effective medical treatment to safely get the baby off of the ventilator machine, and home to her parents, where the baby belongs. Doctors should not play God; they should facilitate patient access to care, not obstruct patient access to care. A patient’s rights should take priority over a physician’s ego.
L’Assurance Maladie’s unfounded rejection is particularly inappropriate now, with Brexit. The cohesion of the EU has become a central issue; each Member State should solemnly respect its obligations vis-à-vis EU law. “Europe is crossing an important milestone,” as you have said. Now is not the time for a leading Member State like France to act as if EU law should apply to other Member States, but not to her. Intentional obstruction, market interference, and non-cooperation is not at all how the EU community is supposed to operate.
Furthermore you have expressed your wish to “totally integrate” the French and German markets, and “restore a strong trust between France and Germany.” L’Assurance Maladie’s senseless refusal to authorise French babies to receive this breakthrough treatment in Germany casts doubt upon France’s commitment to this goal. If France is unwilling to cooperate with Germany to reduce the suffering of newborn French babies who are struggling to breathe, then France will have a very hard time cooperating with Germany on major international projects which are actually divisive and complex.
This case raises an issue of wider principle which matters to all French citizens, and which affects their daily lives: if a newborn French baby suffering from a rare disease, immobilized in an intensive care unit and connected to a ventilator machine, doesn’t have the right to obtain a highly specialised, medically proven and cost-effective treatment for her rare disease in another EU Member State – then who in France does have the right to obtain cross-border medical care?
Mr. President, this document, with its extensive evidence, and letters of support, make it clear that L’Assurance Maladie’s rejection was unfounded. My partner and I urgently request your assistance obtaining Lysiane’s S2, which L’Assurance Maladie illegally and senselessly denied. We hope to hear from you very soon. Thank you.
Sincerely,
Philippe Pakter and █████ ███████ – the parents of Lysiane
2 rue Gérard Maire
69100 Villeurbanne
France
| Letters of Support | page 4 |
| Summary of Dispute | page 20 |
| Background Information | page 22 |
| Medical Analysis | page 33 |
| Legal Analysis | page 62 |
| Conclusion | page 73 |



 Brussels, 18 December 2017
Brussels, 18 December 2017