Rare Revolution Magazine article
RARE Revolution Magazine article, Clinical Spotlight: “Crossing borders to access rare disease care: Lysiane’s Story”

RARE Revolution Magazine article
As seen in the Winter 2019/2020 issue of RARE Revolution Magazine, Issue No. 014
Crossing borders to access rare disease care: Lysiane’s Story
Lysiane is a French baby who was born with a life-threatening rare disease called Pierre Robin Sequence. When the French healthcare system refused to provide Lysiane with authorisation to receive a safe and medically proven rare disease treatment in Germany, her family found themselves at the centre of a challenge for cross-border medicine, which has seen their case go to the top of European law. This is Lysiane’s story.
Written by Philippe Pakter, rare disease parent and patient advocate, supported by Joseph Sadek (intern) and Nicola Miller, RARE Revolution Magazine
“Our patient journey actually began before Lysiane was born. Experts who specialise in my daughter’s rare disease, Pierre Robin Sequence, have identified several known warning signs which appear during the prenatal period, letting doctors know that the baby may be born with Pierre Robin Sequence. Unfortunately, our local hospital, which was not a Centre of Expertise for Pierre Robin Sequence, overlooked all of these known warning signs. Neither my family, nor the delivery room team, were in any way anticipating the birth of a baby with Pierre Robin Sequence. As often happens with these babies, Lysiane was born with an airway emergency. To our shock, Lysiane had to be rushed into the Neonatal Resuscitation Unit, which offers the highest level of critical care. She was hooked up to lots of monitors, cables, tubes and a breathing machine. During one of our visits in this room we saw another baby die. The baby was several beds over from Lysiane’s bed, and it was horrifying. Lysiane remained in her bed, connected to the breathing machine, but my partner and I were told to leave. When we were outside we looked at one another – and at the exact same moment, we both broke down crying. Lysiane could be next. Lysiane was eventually transferred from the Neonatal Resuscitation Unit to the Intensive Care Unit, which for us represented major progress – she made it to the ICU. So much in life is relative; like many rare disease parents out there, we learned this lesson early on.”
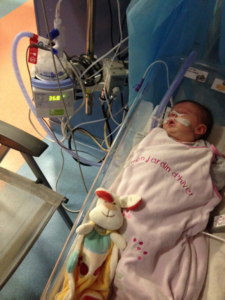
In France, Lysiane, attached to a breathing machine in the Intensive Care Unit. Lysiane remained in the ICU for five straight weeks, without a scheduled date of release.
Pierre Robin Sequence, also known as Pierre Robin Syndrome, is a rare disease which affects approximately one out of 10,000 babies. It is associated with potentially life-threatening breathing difficulties, eating difficulties, oral/facial deformities, central nervous system issues, neurological abnormalities, and other medical problems. Babies with this rare disease face many risks, including oxygen deprivation, brain damage, failure to thrive, and death. Approximately 50% of babies born with Pierre Robin Sequence also suffer from another rare disease or condition, which further increases the complexity and risk. For these reasons, a baby with Pierre Robin Sequence requires a full multidisciplinary team of highly specialised experts, who have experience treating babies with this rare and complex disease.
While Lysiane was in the ICU, connected to a breathing machine, Lysiane’s family carried out intensive research into the rare disease, its symptoms, and possible treatments. Desperate to get Lysiane out of Intensive Care, and wanting to avoid risky and invasive surgery, Lysiane’s family identified a highly specialised, medically proven, non-surgical treatment for Pierre Robin Sequence, developed by a multidisciplinary team of Pierre Robin Sequence experts at the Tübingen University Hospital in Tübingen, Germany. They read numerous medical studies on the German treatment, the Tübingen Palatal Plate, or “TPP treatment”. They contacted the team of German experts behind the TPP treatment, discussed the treatment, and finally decided to visit the German team in person.
“A whole series of internationally peer-reviewed medical studies going back over ten years proves that the TPP treatment safely and effectively resolves the Pierre Robin Sequence baby’s torturous breathing difficulties, frees the baby from the breathing machine, minimises hospitalisation time, and eliminates the need for invasive, risky and painful surgery. Instead of relying on surgery, which should be a last resort for newborn babies, the TPP treatment uses a highly specialised oral medical device, the Tübingen Palatal Plate, which is custom made for each Pierre Robin Sequence baby. We were thrilled to find such evidence – the evidence in evidence-based medicine, which is emphasised so strongly in modern healthcare – was there, making this a viable solution for our daughter. However, it was my family’s in-person visit to the Tübingen University Hospital which really convinced us. We didn’t just meet the highly specialised team of Pierre Robin Sequence experts and ask them countless questions; we also met a young couple who had travelled all the way from Austria so that their newborn baby could receive this TPP treatment in Tübingen. We saw their baby, who had been born just one day before Lysiane. Their baby was sleeping on her back – this is when a Pierre Robin Sequence baby’s breathing is at its absolute worst – and yet this baby was breathing peacefully, freely, effortlessly – it was surreal. And the baby was being discharged from the hospital on that exact same day! Meanwhile Lysiane was back in the French ICU, connected to her breathing machine – and there was no release date in sight. We decided that we wanted Lysiane to receive this TPP treatment. In accordance with EU laws and procedures, we, Lysiane’s parents, both EU citizens, submitted an application to France’s national health insurance system. With no alternative non-invasive treatment available in France, following this consultation, we requested official authorisation, what is called an ‘S2’ form, to permit Lysiane, herself a French citizen, to receive this safe, highly specialised and medically proven TPP treatment in Germany.
The French authorities rejected Lysiane’s application; they claimed that France’s treatment for Pierre Robin Sequence is ‘the same or equally effective’ as Germany’s TPP treatment. At this point we had already had extensive experience with the French treatment, because Lysiane had been receiving this treatment in the French hospital since the day she was born – the breathing machine – the French authorities called this a treatment! Other French treatment options were surgical, including one procedure called labioglossopexy. Labioglossopexy was first proposed a century ago, I mean really, 100 years ago. It is when you sew the baby’s tongue to the baby’s lower lip – all of these were unappealing to us by contrast.”
Denial of the right to cross-border healthcare
In Europe, a sense of community and cohesion is supposed to exist between EU member countries; and for people living in Europe, laws have been passed which clearly guarantee EU citizens the right to access cross-border healthcare, in other European countries. But what happens when those rights are violated?
“The TPP treatment is time sensitive; a baby with Pierre Robin Sequence should wear the TPP device in her mouth for a total of 3 to 5 months, and then never has to wear it again. It is a one-off treatment. The catch is that this period of wearing the TPP device has to begin shortly after the baby is born. My family and I knew that if we spent several months arguing with the French bureaucrats, Lysiane would forever lose the opportunity to benefit from the TPP treatment. We’re not wealthy, so at this point my father, Lysiane’s grandfather, took out a bank loan, using his home as the security for the loan, and sent the money to the German hospital. We then transferred Lysiane from France, to Germany, to receive the TPP treatment, paying for the treatment using this bank loan. Lysiane received the TPP treatment, which, as promised, forever liberated her from the breathing machine. Since she no longer needed a breathing machine, we could finally leave the hospital, take her home, bring her to the park, go with her to the supermarket, visit her grandparents and relatives, everything – total mobility, a radically improved quality of life. Finally Lysiane was receiving the successful, short-term, non-invasive, TPP Treatment!”

Lysiane feeling festive and getting ready for Christmas, free from intrusive tubes and monitors and home with her family.
Appealing the refusal
Lysiane’s parents are appealing the French government’s refusal to authorise the medically proven TPP treatment in Germany. They have built up a strong set of allies. In one of the many letters of support they have received, a rare disease patient group in Scandinavia described Lysiane’s case as “a devastating example of the struggle that many rare disease patients and families have faced, and continue to face, right here in Europe”. A senior Member of European Parliament from France wrote an official letter to the French Health Minister, Agnès Buzyn, calling the refusal “unacceptable”, and legally unfounded. The European Patients’ Forum, the largest patient advocacy umbrella organization in the entire EU, sent a formal letter of support directly to the French President, Emmanuel Macron. In their letter they state that this situation is “shocking”, and that France’s rejection was legally unfounded. EURORDIS, one of the most important rare disease patient organization in the world, has pledged to accompany Lysiane’s parents in court, all the way up to the Court of Justice of the European Union. And the European Commission’s SOLVIT Network, after carefully analysing the case, formally concluded that the French authorities are in violation of EU law, both the 2011 Cross-border Healthcare Directive, and Regulation 883 (SOLVIT Case Number 2569/17/DE).
Philippe, Lysiane’s father, about his next steps
“This challenging rare disease patient journey has turned me into a rare disease patient advocate. I established a non-profit organisation to raise awareness of this rare disease, Pierre Robin Sequence, and to promote the rights of these babies. This year I’ve taken several trips to Brussels, where I’ve had individual face to face meetings with Members of European Parliament. I’ve successfully organised a multi-party coalition of Members of European Parliament from every major political party in the EU: the EPP, S&D, Renew Europe, and the Greens. This multi-party coalition in European Parliament, together with EURORDIS, the European Patients Forum, EU law professors, healthcare policy experts, and various rare disease patient groups, will help my family and other Pierre Robin Sequence parents to finally achieve justice. The TPP is not some new, uncertain, experimental treatment; the TPP’s effectiveness and safety have been definitively proven in numerous internationally peer-reviewed medical studies, going back over ten years. Babies from other EU member countries have been transferred to Germany to receive the TPP treatment, including babies from low income European member countries. Babies from Russia have been transferred to Germany to receive the TPP treatment. Even babies from as far away as the west coast of the United States of America have been transferred, over enormous distances, to Tübingen Germany, to receive this breakthrough treatment.
Next year an important medical conference will be held, bringing together Pierre Robin Sequence experts from all over the entire world. The international physicians who organise this conference chose to hold the event at the Tübingen University Hospital, the birthplace of the TPP treatment. No objective and impartial medical comparison can reasonably conclude that France’s treatment is ‘the same or equally effective’ as the TPP treatment.
We know it will take time, but France will eventually have to reverse this rejection – either voluntarily, or by the order of the Court of Justice of the European Union. Meanwhile, we believe that Lysiane remains the only French baby to ever be transferred across that one single border, to Germany, to benefit from this medical breakthrough in Tübingen. Innocent babies paying the price for senseless obstruction – it really is quite sad and beyond frustrating.”
Philippe believes that the experience he and his family are facing in France points to a widespread problem faced by many patients throughout the world – deliberate resistance, non-cooperation and outright obstruction, blocking patients, including rare disease patients, from accessing highly specialised care. According to Philippe, this known and serious problem was officially acknowledged and formally documented earlier this year in the European Parliament’s Implementation Report on the 2011 Directive, Report A8-0046/2019.
“Problems can arise out of administrative incompetence – a failure to understand the laws governing EU cross-border healthcare. But problems can also result from competition between doctors, a generalist’s reluctance to lose patients to a specialist, even from pride itself: a physician’s inability to admit that another doctor may have more knowledge, or may have developed a better treatment. Whatever the reason, it comes down to this: nowhere is the logic or moral imperative for cross-border healthcare more obvious than it is for rare disease patients seeking a highly specialised treatment which is not available in their own member countries – and yet this is the exact same patient group which is most likely to run into obstacles to cross-border care.
Based on the principle that the true measure of any society can be found in how it treats its most vulnerable members, the disproportionate harm which this ongoing obstruction inflicts on rare disease patients is absolutely shameful. There is no place for this in 21st century Europe. Lysiane’s case raises an issue of wider principle which matters to all European citizens, and which affects their daily lives: if a newborn baby suffering from a rare disease, immobilized in an Intensive Care Unit and connected to a breathing machine, doesn’t have the right to a highly specialised, medically proven, safe, and cost-effective treatment for her rare disease in another EU member country – then who DOES have the right to cross-border care?”











