
Podcast interview with Pierre Robin Sequence parent
Osmosis/Elsevier’s “Raise the Line” Podcast interviews the parent of a child with the rare disease, Pierre Robin Sequence: “What rare disease patients need from clinicians.”

The 3rd International Pierre Robin Sequence Consensus Meeting
A rare disease parent speaks candidly with clinicians about the rare disease patient's point of view. Evidence-based medicine; access to care; medical ethics; and quality of life.

About us without us
Zero PRS patients in the working group, and zero PRS patients in the consensus vote, for ERN-Cranio's EU-funded Guidelines for the rare disease, PRS... about us, without us.

Lysiane
A letter to France's President Macron about a French baby with a rare disease who was denied access to a safe and effective PRS treatment in Germany.
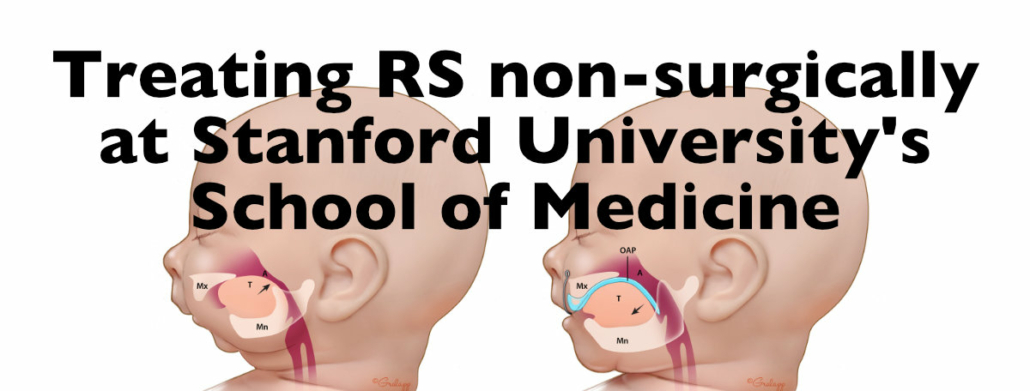
Dr. HyeRan Choo and the non-surgical Orthodontic Airway Plate treatment at Stanford
Dr. HyeRan Choo, Clinical Assistant Professor of Plastic and Reconstructive Surgery at Stanford University School of Medicine, speaks with us about the breakthrough non-surgical treatment she and her team offer to Pierre Robin Sequence babies at Lucile Packard Children’s Hospital Stanford in Palo Alto, California.

ERN-Cranio’s EU Clinical Practice Guidelines for Pierre Robin Sequence
When EU Guidelines for a rare disease are officially rejected by the EU's top two experts on that rare disease, then the EU Guidelines are nonviable.

A parent’s view on the care of their baby with Pierre Robin Sequence
A parent's view on the care of their baby with Pierre Robin Sequence. Published in "Seminars in Fetal & Neonatal Medicine".
Prenatal Diagnosis of Pierre Robin Sequence: an Interview with Dr. Cory Resnick
Dr. Cory M. Resnick, Oral & Maxillofacial Surgeon at Boston Children's Hospital and Assistant Professor at Harvard Medical School, describes the exciting progress he has made on the Prenatal Diagnosis of Pierre Robin Sequence.

Letter to EU Health Commissioner Kyriakides
EU Health Commissioner Stella Kyriakides: this situation raises an issue of wider principle which matters to all EU citizens, and which affects their daily lives: if a newborn baby suffering from a rare disease, immobilized in an intensive care unit and connected to a breathing machine, doesn’t have the right to obtain a highly specialised, medically proven, safe, and cost-effective treatment for her rare disease in another EU Member State – then who DOES have the right to cross-border care?

Rare Revolution Magazine article
RARE Revolution Magazine article, Clinical Spotlight: “Crossing borders to access rare disease care: Lysiane’s Story”

Pierre Robin Sequence – Information Document for Parents
Although Pierre Robin Sequence is described in Europe's Orphanet Rare Disease Database, and in America's NORD Rare Disease Database, and in the GARD Genetic and Rare Diseases Database, the information provided in these databases seems to be written for physicians, and not for parents. Our goal was to produce the kind of document each of us wishes we as parents could have had, when the doctors first told us that our baby had Pierre Robin Sequence.

European Parliament’s Implementation Report on the 2011 Cross-border Healthcare Directive
In a time of growing populist movements, deepening economic uncertainty, and foreboding Euroscepticism, the EU’s 2011 Cross-border Healthcare Directive was intended to serve as a prime example of the real and tangible benefits which the European Union could bring to its citizens. Leveraging the power of Europe’s common market, and solidly grounded in the EU’s four fundamental freedoms – including the free movement of goods and services, and the free movement of people – the 2011 Directive boldly declared that EU citizens had the right to access medical services anywhere in Europe, without facing undue resistance, obstacles, obstruction or delay...

Rare Disease Collaboration… and Obstruction
Even if global policies support rare diseases, all the way up to the UN level - and even if various tools have been created to promote the rare disease agenda - in Europe I am thinking of Orphanet, Clinical Patient Management Systems, European Reference Networks, the 2011 Cross-border Health Directive, etc... what should rare disease patients do when local doctors do not want to cooperate?

Rare Diseases Denmark – letter of support
Rare Diseases Denmark, a leading rare disease advocacy group from Scandinavia, is a highly respected voice in the international rare disease movement. In their letter of support, they point out that the case of Lysiane "is a devastating example of the struggle that many rare disease patients and families have faced, and continue to face, right here in Europe."

Pro Rare Austria – letter of support
Pro Rare Austria took center stage at the European Conference on Rare Diseases which was recently held in Vienna. As explained by Pro Rare Austria’s Chairman, Dr. Rainer Riedl, "With rare diseases, the sharing of knowledge, the exchange of expertise, as well as networking and cooperation, are particularly important." In their letter to France's L'Assurance Maladie, they ask the Director, Nicolas Revel, to revise the unfounded rejection of Lysiane's right to a safe and medically proven treatment in Germany.

A third letter to President Macron
On 12 October 2018 we sent a third letter to French President Emmanuel Macron...

European Patients Forum – letter of support
The European Patients Forum is the largest patient advocacy umbrella organization in Europe. They wrote this letter below, and they mailed it, directly to France's President, President Macron.

Françoise Grossetête, Member of European Parliament – letter of support
Ms. Françoise Grossetête, a senior Member of European Parliament, has a background in law - and Ms. Grossetête personally served as the European Parliament’s Special Rapporteur for the EU’s 2011 Directive on Cross-border Healthcare. Ms. Grossetête knows cross-border healthcare law. On our daughter Lysiane's behalf, she sent the letter below directly to France’s Minister of Health, Agnès Buzyn, making it clear that this situation is "unacceptable", and that the rejection was legally unfounded.

EURORDIS – letter of support
The European Organisation for Rare Diseases (EURORDIS) is the largest rare disease patient organization in the EU. EURORDIS wrote a highly detailed letter of support to L’Assurance Maladie, France's national health insurance fund, explaining why they too believe the rejection violated EU law. EURORDIS has pledged to accompany us, Lysiane’s parents, in court, all the way up to the European Court of Justice.











